
Concept explainers
(a)
Interpretation:
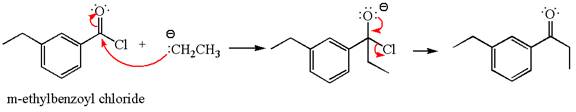
The product with detailed mechanism for the reaction between
Concept introduction:
An acid chloride can be converted to corresponding
Answer to Problem 20.45P
The product with detailed mechanism for the reaction between
Explanation of Solution
The equation for the reaction of
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(b)
Interpretation:
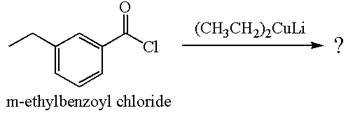
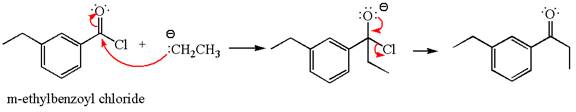
The product with detailed mechanism for the reaction between
Concept introduction:
An acid chloride can be converted to an
Answer to Problem 20.45P
The product with detailed mechanism for the reaction between
Explanation of Solution
The equation for the reaction of
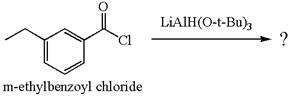

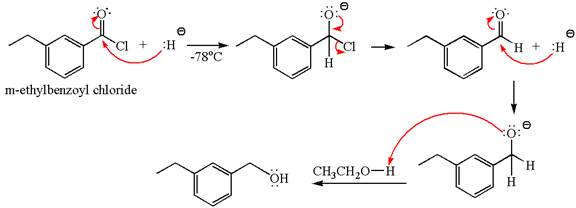
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(c)
Interpretation:
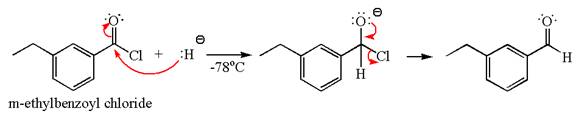
The product with detailed mechanism for the reaction between
Concept introduction:
An acid chloride can be converted to a primary alcohol by reacting with
Answer to Problem 20.45P
The product with detailed mechanism for the reaction between
Explanation of Solution
The equation for the reaction of
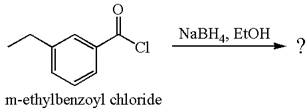
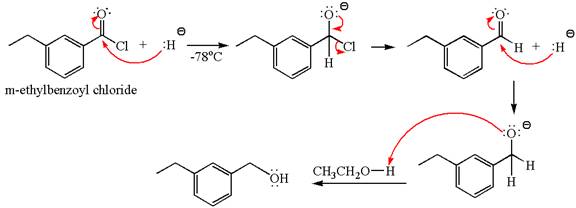
The product with detailed mechanism for the given reaction is drawn based on the reactivity of
(d)
Interpretation:
The product with detailed mechanism for the reaction between
Concept introduction:
An acid chloride can be converted to a tertiary alcohol by reacting it with
Answer to Problem 20.45P
The product with detailed mechanism for the reaction between
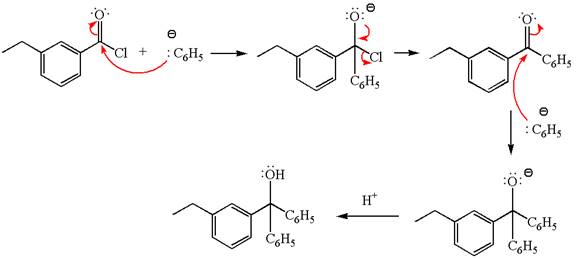
Explanation of Solution
The equation for the reaction of
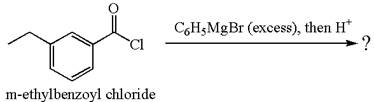

The product with detailed mechanism for the given reaction is drawn based on the reactivity of
Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- A. Arrange the following radicals in order of decreasing rate of bromination. Justify your answer. B. Trehalose and isomaltose are both dimers of glucose. However, they have considerablydifferent reactivities. Concisely explain why these differences are observed. -Isomaltose is a reducing sugar while trehalose is not.-Trehalose is very resistant to acid hydrolysis while isomaltose can be acid-hydrolyzed withease.arrow_forwardGive the major product of the following reaction for letter d) and explain the mechanism/processarrow_forwardgive the mechanism of the following reactions and explain why any selectivities occur.arrow_forward
- provide the mechanism for the following reaction. only typed solutionarrow_forwardProvide the mechanism for the following reactions.arrow_forwardProvide the synthesis for the following product starting with the provide starting material. More than one step is required. Show the mechanism for each reaction. Please give details.arrow_forward
