
Concept explainers
a)
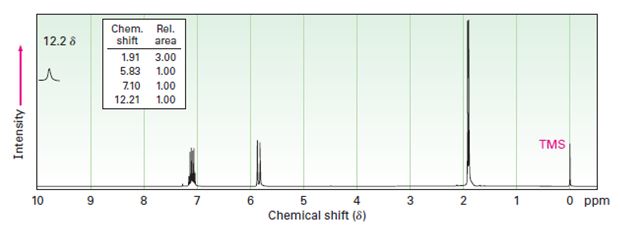
Interpretation:
Two 1HNMR spectra belonging to crotonic acid (trans-CH3CH=CHCOOH) and methacrylic acid (H2C=C (CH3) COOH) are given. Which corresponds to which is to be explained.
1HNMR: Spectrum (a): 1.91 δ (Rel.area: 3.00), 5.83 δ (Rel.area: 1.00), 7.10 δ (Rel.area: 1.00), 12.21 δ (Rel.area: 1.00).
Concept introduction:
Crotonic acid (trans-CH3CH=CHCOOH) and methacrylic acid (H2C=C (CH3) COOH) can be easily distinguished by looking the signal for the methyl protons in these two compounds.
To explain:
Of the two 1HNMR spectra given, which belongs to crotonic acid (trans-CH3CH=CHCOOH) and methacrylic acid (H2C=C (CH3) COOH).
b)
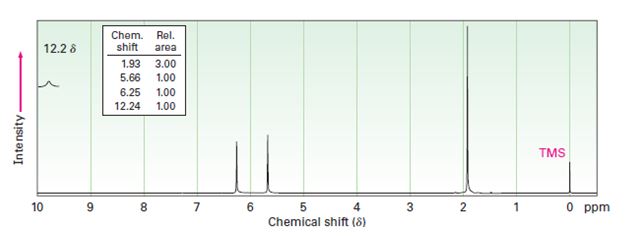
Interpretation:
Two 1HNMR spectra belonging to crotonic acid (trans-CH3CH=CHCOOH) and methacrylic acid (H2C=C (CH3) COOH) are given. Which corresponds to which is to be explained.
1HNMR: Spectrum (b): 1.93 δ (Rel.area: 3.00), 5.66 δ (Rel.area: 1.00), 6.25 δ (Rel.area: 1.00), 12.24 δ (Rel.area: 1.00).
Concept introduction:
Crotonic acid (trans-CH3CH=CHCOOH) and methacrylic acid (H2C=C (CH3) COOH) can be easily distinguished by looking the signal for the methyl protons in these two compounds.
To explain:
Of the two 1HNMR spectra given, which belongs to crotonic acid (trans-CH3CH=CHCOOH) and methacrylic acid (H2C=C (CH3) COOH).
Trending nowThis is a popular solution!

Chapter 20 Solutions
Organic Chemistry
- Compound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forward3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardC9H10O2 analyze the structure from the 1H nmr dataarrow_forward
- A solution of acetone [(CH3)2C=O] in ethanol (CH3CH2OH) in the presence of a trace of acid was allowed to stand for several days, and a new compound of molecular formula C7H16O2 was formed. The IR spectrum showed only one major peak in the functional group region around 3000 cm−1, and the 1H NMR spectrum is given here. What is the structure of the product?arrow_forwardAs we will learn in Chapter 17, reaction of (CH3)2CO with LIC≡CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600−3200, 3303, 2938, and 2120 cm−1. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?arrow_forwardThis question relates to the1H-NMR spectrum of alkenes. How many proton environments are there in propene? How many proton environments are there in trans-2-butene? What are the multiplicities of each proton environment in trans-2-butene? What are the multiplicities of each proton environment in propene?arrow_forward
- Predict the ¹H NMR spectrum of diethoxymethane.arrow_forwardAs we will learn in Chapter 20, reaction of (CH3)2CO with LiC ≡ CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600–3200, 3303, 2938, and 2120 cm. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

