
(a)
Interpretation:
The product of the reaction between
Concept introduction:
The carbonyl carbon of the acid chloride is highly electrophilic and shows nucleophilic addition-elimination reaction with the nucleophile. In the first step, the nucleophile adds the electron deficient carbonyl carbon; it is known as the nucleophilic addition step. Since the chlorine atom is a good leaving group, it departs from the substrate in a nucleophilic elimination step.
Answer to Problem 21.36P
The product of the given reaction is
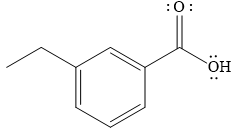
The complete detailed mechanism for the given reaction is
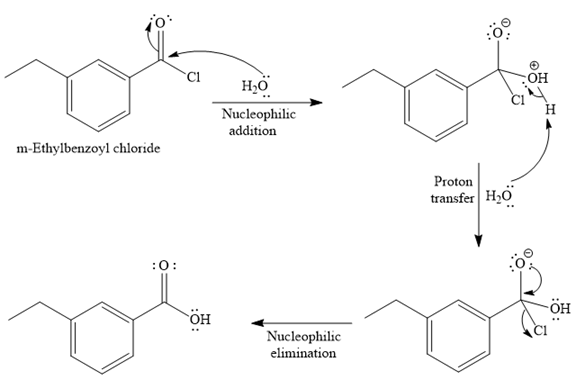
Explanation of Solution
The given reaction is
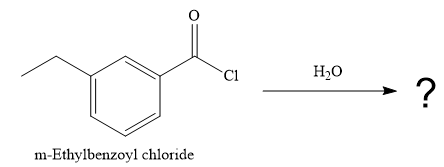
Since water is a nucleophile, it attacks the electron deficient carbonyl carbon of
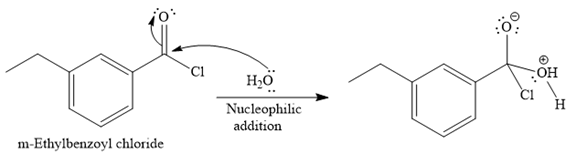
In the second step, the positive charge of the water’s oxygen is deprotonated, and simultaneously the chlorine anoin departs from the substrate with its bonding pair. Thus, it is called a nucleophilic elimination.
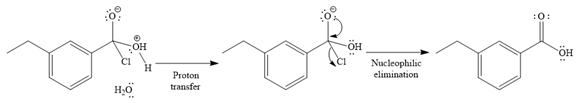
The product of the reaction between
(b)
Interpretation:
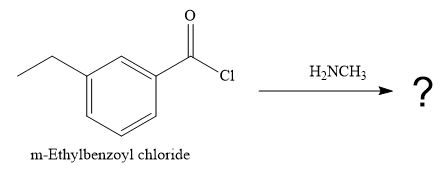
The product of the reaction between
Concept introduction:
The carbonyl carbon of the acid chloride is highly electrophilic and shows nucleophilic addition-elimination reaction with the nucleophile. In the first step, the nucleophile adds the electron deficient carbonyl carbon; it is known as the nucleophilic addition step. Since the chlorine atom is a good leaving group, it departs from the substrate in a nucleophilic elimination step.
Answer to Problem 21.36P
The product of the given reaction is
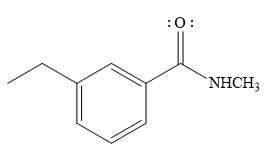
The complete detailed mechanism for the given reaction is
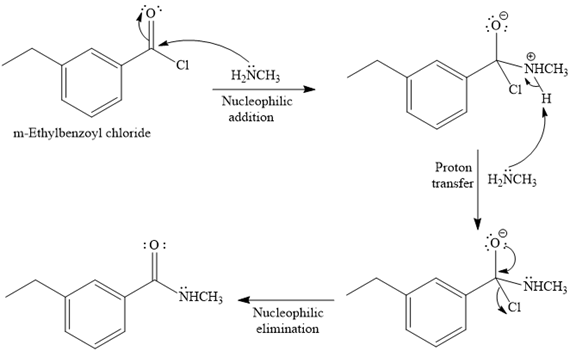
Explanation of Solution
The given reaction is
Since methyl
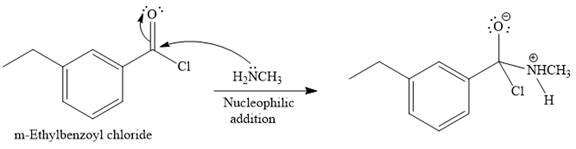
In the second step, the positive charge of the nitrogen atom is deprotonated, and simultaneously chlorine atom departs from the substrate with its bonding pair in a nucleophilic elimination step.
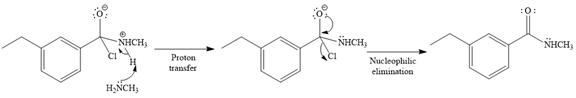
The product of the reaction between
(c)
Interpretation:
The product of the reaction between
Concept introduction:
The carbonyl carbon of the acid chloride is highly electrophilic and shows nucleophilic addition-elimination reaction with the nucleophile. In the first step, the nucleophile adds the electron deficient carbonyl carbon; it is known as the nucleophilic addition step. Since the chlorine atom is a good leaving group, it departs from the substrate in a nucleophilic elimination step.
Answer to Problem 21.36P
The product of the given reaction is
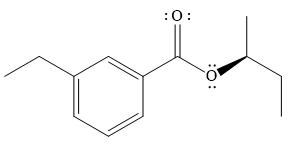
The complete detailed mechanism for the given reaction is
Explanation of Solution
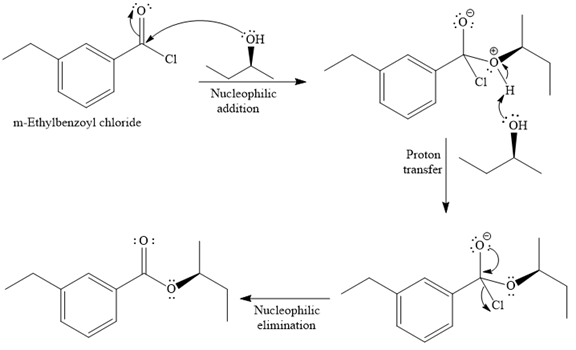
The given reaction is
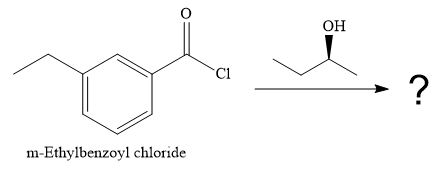
Since an alcohol is a nucleophile, it attacks the electron deficient carbonyl carbon of
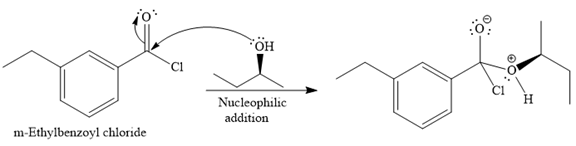
In the second step, the positive charge of the alcohol’s oxygen is deprotonated, and simultaneously the chlorine anion departs from the substrate with its bonding pair. Thus, it is called a nucleophilic elimination.
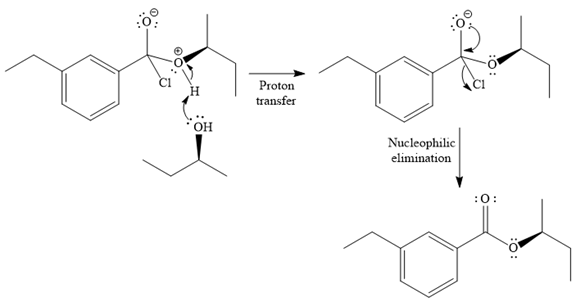
The product of the reaction between
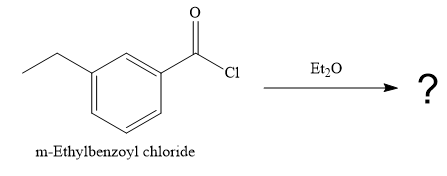
(d)
Interpretation:
The product of the reaction between
Concept introduction:
The carbonyl carbon of the acid chloride is highly electrophilic and shows nucleophilic addition-elimination reaction with the nucleophile. In the first step, the nucleophile adds the electron deficient carbonyl carbon; it is known as the nucleophilic addition step. Since the chlorine atom is a good leaving group, it departs from the substrate in a nucleophilic elimination step.
Answer to Problem 21.36P
No reaction occurs as the positive charge of the oxygen atom is not simply deprotonated.
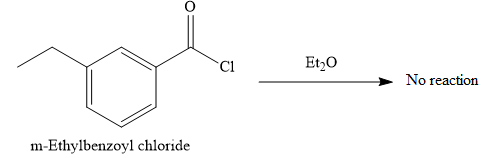
Explanation of Solution
The given reaction is
When the lone pair of the oxygen atom attacks the carbonyl carbon, it acquires a positive charge, which does not show deprotonation reaction.
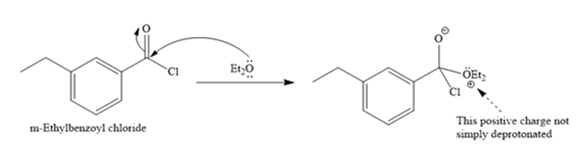
Thus, the given reaction does not occur.

No reaction occurs as the positive charge of the oxygen atom is not simply deprotonated.
Want to see more full solutions like this?
Chapter 21 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





