
Interpretation:
The reagent has to be given for the conversion of A to Tamoxifen.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as
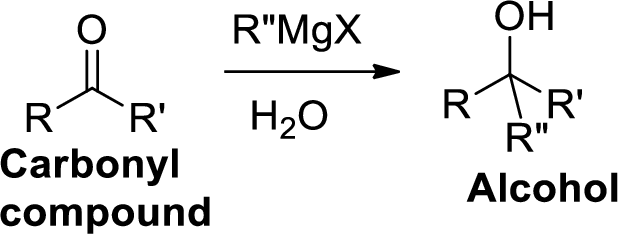
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
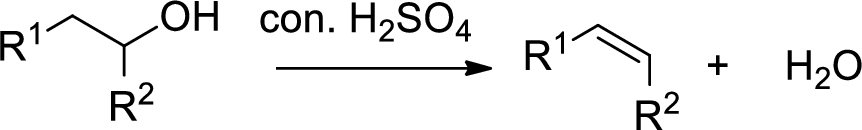
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.
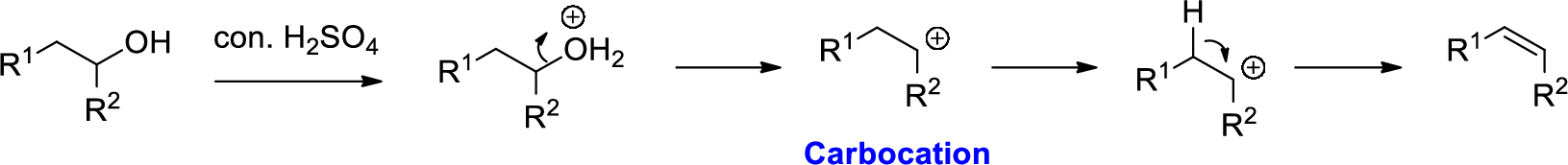
In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- 4. Which effect does an antagonistic drug have? (a) It inhibits the activity of an enzyme (b) It promotes the activity of an enzyme (c) It inhibits the signalling of a receptor (d) It promotes the signalling of a receptor (e) It promotes nerve signalling 7. Which technique is not used for structural analyses of possible lead compounds? (a) NMR (b) IR (c) X-Ray crystallography (d) MRI (e) MSarrow_forwardWhy is methyl salicylate so easily absorbed through the skin?arrow_forwardParoxetine (Paxil) is an antidepressant that is a member of a family of drugs known as Selective Serotonin Reuptake Inhibitors (SSRIS). This family of drugs also includes fluoxetine (Prozac) and sertraline (Zoloft). SSRIS work by inhibiting the reuptake of the neurotransmitter serotonin in the synapses of the central nervous system follow- ing release of serotonin during excitation of individual nerve cells. Between firings, the serotonin is taken back up by a nerve cell in preparation for firing again. Inhibition of reuptake has the effect of increasing the time serotonin molecules remain in the syn- apses following excitation, leading to a therapeutic effect. In one synthesis of parox- etine, the following reagents are used. Draw the structures of synthetic intermediates A and B. F НО SOCI, A B HO Pyridinearrow_forward
- (i)What class of drug is Ranitidine? (ii)If water contains dissolved Ca2+ ions, out of soaps and synthetic detergents, which will you use for cleaning clothes? (iii)Which of the following is an antiseptic? 0.2% phenol, 1% phenolarrow_forwardNitriles can be formed by treating primary and secondary amides secondary amines primary amines with SOCl2? tertiary aminesarrow_forwardFrom the given structures which is(a) amide that will release a secondary amine upon hydrolysis? (b) product of hydrolysis of MSO (c) a tertiary amide and (d) a diketonearrow_forward
- Compounds like amphetamine that contain nitrogen atoms are protonated by the HCl in the gastric juices of the stomach, and the resulting salt is then deprotonated in the basic environment of the intestines to regenerate the neutral form. Write proton transfer reactions for both of these processes.arrow_forward¨ What other types of analgesics are on the market? How does their action compare with aspirin and why are these alternative drugs necessary? Discuss the differences among the following compounds by drawing their structures: Acetylsalicylic acid, Acetaminophen (Paracetamol), Ibuprofen, Paracetamol and Phenacetin.arrow_forwardUsing the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forward
- Which of the following are descriptions of possible starting material for this reaction? ? trace acid an ester a ketone an imine an aldehyde a carboxylic acid an enamine a primary amine a secondary amine a tertiary amine H Iarrow_forwardSelect two substrates and one reagent from each of the given pools to create the amine target molecule.arrow_forward17-35 Suppose that you take a bottle of benzaldehyde (a liquid, bp 179°C) from a shelf and find a white solid in the bottom of the bottle. The solid turns litmus red; that is, it is acidic. Yet aldehydes are neutral compounds. How can you explain these observations?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



