
(a)
Interpretation:
The synthesis of carbinoxamine has to be shown.
Concept introduction:
The Grignard reaction:
Alkyl, vinyl, or aryl-magnesium halides (
Grignard reagent is reaction with carbonyl compound such as
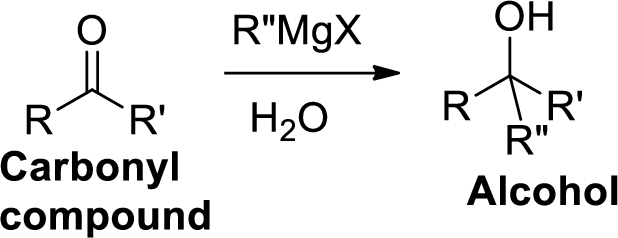
(b)
Interpretation:
The chirality of Carbinoxamine is to be identified and the possible stereoisomers has to be identified in the given synthesis.
Concept introduction:
Chiral:
A molecule is non superimposable on its mirror image is called chiral molecule.
Four different atoms attached to a carbon atom is called chiral molecule.
Isomer:
A molecule having the same molecular formula but with different chemical structure is called isomer.
Stereoisomers:
Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers:
A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers:
A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture:
A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Verapamil, a coronary artery vasodilator, is used in the treatment of angina caused by insufficient blood flow to cardiac muscle. Even though its effect on coronary vasculature tone was recognized over 30 years ago, only recently has its role as a calcium channel blocker become understood. Following is a retrosynthetic analysis leading to a convergent synthesis; it is convergent because (A) and (B) are made separately and then combined (i.e., the route converges) to give the final product. Convergent syntheses are generally much more efficient than those in which the skeleton is built up stepwise. Q. Two steps are required to convert (D) to (C). The first is treatment of (D) with ethyl chloroformate. What is the product of this first step? What reagent can be used to convert this product to (C)?arrow_forwardVerapamil, a coronary artery vasodilator, is used in the treatment of angina caused by insufficient blood flow to cardiac muscle. Even though its effect on coronary vasculature tone was recognized over 30 years ago, only recently has its role as a calcium channel blocker become understood. Following is a retrosynthetic analysis leading to a convergent synthesis; it is convergent because (A) and (B) are made separately and then combined (i.e., the route converges) to give the final product. Convergent syntheses are generally much more efficient than those in which the skeleton is built up stepwise. Q. How do you account for the regioselectivity of the nucleophilic displacement involved in converting (C) to (B)?arrow_forwardRank each set of compounds in order of increasing basicity.pyrrole, imidazole, 3-nitropyrrolearrow_forward
- (b) Rank the following compounds in order of decreasing ease of removing a proton from amethyl group. Provide an explanation CH3 CH3 CH3 CH2Carrow_forwardRank each set of compounds in order of increasing basicity.aniline, p-methylaniline, p-nitroanilinearrow_forwardPredict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanol (b) sodium acetate (c) anilinearrow_forward
- The analgesic Tylenol is often taken by people who are allergic to aspirin. Tylenol contains acetaminophen (structure shown) as the active ingredient. Is the structure of acetaminophen similar to the structure of aspirin? In what way? Would acetaminophen give a positive phenol test? What products would be obtained if acetaminophen were hydrolyzed in acidic aqueous solution?arrow_forwardThe –NHCOR group of an amide is an activating group, but it is not as strongly activating as NH2. (a) Explain why it is an activating group. (b) Explain why it is less activating than NH2.arrow_forwardi need the answer quicklyarrow_forward
- Mustard gas, Cl¬CH2CH2¬S¬CH2CH2¬Cl, was used as a poisonous chemical agentin World War I. Mustard gas is much more toxic than a typical primary alkyl chloride. Itstoxicity stems from its ability to alkylate amino groups on important metabolic enzymes,rendering the enzymes inactive.(a) Propose a mechanism to explain why mustard gas is an exceptionally potent alkylatingagentarrow_forwardThe (R)-isomer of a-terpineol is a component of perfumes and flavorings and has a lilac-like floral odor. The (S)-isomer has a pine-like odor. Propose two methods of producing a-terpineol using Grignard reactions. [Ignore stereochemistry in your synthesises.]arrow_forwardThe Stork reaction is a condensation reaction between an enamine donor and an α,β-unsaturated carbonyl acceptor. The overall reaction consists of a three-step sequence of formation of an enamine from a ketone, Michael addition to an α,β-unsaturated carbonyl compound, and hydrolysis of the enamine in dilute acid to regenerate the ketone. Consider the Stork reaction between acetophenone and 3-buten-2-one. Draw the structure of the product of the enamine formed between acetophenone and pyrrolidine. Draw the structure of the Michael addition product. Draw the structure of the final product.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


