
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.64SP
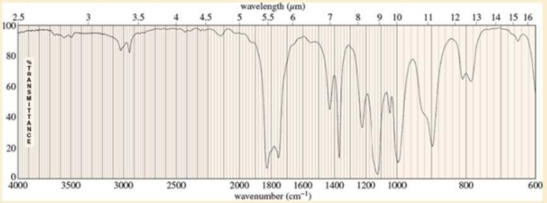
A chemist was called to an abandoned aspirin factory to determine the contents of a badly corroded vat. Knowing that two salvage workers had become ill from breathing the fumes, she put on her breathing apparatus as soon as she noticed an overpowering odor like that of vinegar but much more pungent. She entered the building and took a sample of the contents of the vat. The mass spectrum showed a molecular weight of 102, and the NMR spectrum showed only a singlet at δ2.15. The IR spectrum, which appears here, left no doubt about the identity of the compound. Identify the compound, and suggest a method for its safe disposal.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An aromatic compound K, whose molecular formula is C8H11N, is examined in the laboratory to elucidate its structure. The following observations were made:
A) Compound K is soluble in dilute hydrochloric acid but insoluble in sodium hydroxide solution.
B) Treatment of compound K with excess potassium hydroxide and benzenesulfonyl chloride, C(6)H(5)SO(2)Cl, results in the formation of a heterogeneous mixture. The NMR spectrum of compound K is shown below.
C) Compound K when treated with acetic anhydride[CH3-C(O)-O-C(O)-CH3], gives compound L, whose molecular formula is C(10)H(13)ON. Compound L is insoluble in dilute acid or dilute base at room temperature, heating compound L in dilute acid or base, however, regenerates compound K.
D) When compound L is heated with a mixture of concentrated nitric acid and sulfuric acid, a single product, compound M, with the molecular formula C(10)H(12)O(3)N(2) is formed in excellent yields.
On the basis of these observations draw the structures of…
identify the compound with molecular formula C2H6O that gives this 1H NMR spectrum.
Choose the structure corresponding to the given 1H and 13C NMR spectra
Chapter 21 Solutions
Organic Chemistry (9th Edition)
Ch. 21.2F - Name the following carboxylic acid derivatives,...Ch. 21.4A - Prob. 21.2PCh. 21.4A - Prob. 21.3PCh. 21.4A - Prob. 21.4PCh. 21.5C - Prob. 21.7PCh. 21.6 - When ethyl 4-hydroxybutyrate is heated in the...Ch. 21.6 - Propose a mechanism for the following ring-opening...Ch. 21.6 - Prob. 21.15PCh. 21.7B - Prob. 21.16PCh. 21.7C - Prob. 21.19P
Ch. 21.7C - Prob. 21.20PCh. 21.7C - Prob. 21.21PCh. 21.7D - Prob. 21.22PCh. 21.7D - The mechanism for acidic hydrolysis of a nitrile...Ch. 21.8A - Prob. 21.24PCh. 21.8C - Prob. 21.25PCh. 21.9 - Prob. 21.26PCh. 21.9 - Prob. 21.27PCh. 21.9 - Prob. 21.28PCh. 21.10 - Draw a mechanism for the acylation of anisole by...Ch. 21.10 - Prob. 21.30PCh. 21.11 - Prob. 21.31PCh. 21.11 - Prob. 21.32PCh. 21.12 - Problem 21-33 Propose a mechanism for the...Ch. 21.12 - Suggest the most appropriate reagent for each...Ch. 21.12 - Show how you would synthesize each compound,...Ch. 21.13 - Prob. 21.36PCh. 21.13 - Prob. 21.37PCh. 21.14 - Prob. 21.38PCh. 21.14 - Prob. 21.39PCh. 21.16 - Prob. 21.40PCh. 21.16 - Prob. 21.41PCh. 21 - Prob. 21.42SPCh. 21 - Give appropriate names for the following...Ch. 21 - Predict the major products formed when benzoyl...Ch. 21 - Predict the products of the following reactions....Ch. 21 - Prob. 21.46SPCh. 21 - Prob. 21.47SPCh. 21 - Prob. 21.48SPCh. 21 - Propose mechanisms for the following reactions.Ch. 21 - Prob. 21.51SPCh. 21 - An ether extraction of nutmeg gives large...Ch. 21 - Prob. 21.53SPCh. 21 - Show how you would accomplish the following...Ch. 21 - Prob. 21.55SPCh. 21 - Prob. 21.56SPCh. 21 - Prob. 21.57SPCh. 21 - Prob. 21.58SPCh. 21 - Prob. 21.59SPCh. 21 - Explain this curious result. What does this...Ch. 21 - Prob. 21.61SPCh. 21 - Prob. 21.62SPCh. 21 - Prob. 21.63SPCh. 21 - A chemist was called to an abandoned aspirin...Ch. 21 - Prob. 21.67SPCh. 21 - The IR spectrum, 13ONTVTR spectrum, and 1HNMR...Ch. 21 - Prob. 21.69SPCh. 21 - Prob. 21.70SPCh. 21 - Prob. 21.71SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compound A is a hydrocarbon with a molar mass of 96g/mol, with the given C13 spectral data. When compound A reacts with BH3 followed by the treatment with basic H2O2 it is converted to compound B. Propose structures for A and B, explain your analysis.Compound A- Proton decoupled C NMR: 26.8, 28.7, 35.7, 106.9, 149.7 δ.DEPT-90: No peak.DEPT-135: No positive peaks; negative peaks at 26.8, 28.7, 35.7, 106.9 δ.Compound B- Proton decoupled C NMR: 26.1, 26.9, 29.9, 40.5, 68.2 δ.DEPT-90: 40.5 δ.DEPT-135: positive peak at 40.5 δ; negative peaks at 26.1, 26.9, 29.9, 68.2 δarrow_forward(a) Compound A has molecular formula C5H10O. It shows three signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.1 ppm, a singlet of integral 3 at 2.14 ppm, and a quintet of integral 1 at 2.58 ppm. Suggest a structure for A and explain your reasoning. (b) Compound B has molecular formula C8H6O2. The IR, 1H-NMR, and 13C-NMR spectra are shown below, they are also downloadable for closer inspection by clicking the link under the spectral data. Suggest a structure for B and explain your reasoning. (c) Compound C has molecular formula C5H8O. The IR, mass, 1H-NMR, and 13C-NMR spectra are shown below, they are also downloadable for closer inspection by clicking the link under the spectral data. Suggest a structure for C and explain your reasoning.arrow_forwardA hydrocarbon, compound B, has molecular formula C6H6, and gave an NMR spectrum with two signals: delta 6.55 pm and delta 3.84 pm with peak ratio of 2:1. When warmed in pyridine for three hr, compound B quantitatively converts to benzene. Mild hydrogenation of B yielded another compound C with mass spectrum of m/z 82. Infrared spectrum showed no double bonds; NMR spectrum showed one broad peak at delta 2.34 ppm. With this information, address the following questions. a) How many rings are in compound C? b) How many rings are probably in B? How many double bonds are in B? c) Can you suggest a structure for compounds B and C? d) In the NMR spectrum of B, the up-field signal was a quintet, and the down field signal was a triplet. How must you account for these splitting patterns?arrow_forward
- Propose a structure for an organic compound with structural formula C10H13NO2 based on the given IR and 1H NMR spectra. Explain your answer by interpreting the spectraarrow_forwardAn unknown compound has the molecular formula C7H14O, and its 1H NMR and 13C NMR spectra are shown below. Determine the structure of the unknown compound and draw it below. Note that there are no peaks above 3 ppm in the 1H NMR, and the numbers present on the 1H NMR are the integration values for each set of peaks.arrow_forwardFollowing are 1H-NMR spectra for compounds B (C6H12O2) and C (C6H10O). Upon warming in dilute acid, compound B is converted to compound C. Deduce the structural formulas for compounds B and C.arrow_forward
- Following is the 1H-NMR spectrum of compound O, molecular formula C7H12. Compound O reacts with bromine in carbon tetrachloride to give a compound with the molecular formula C7H12Br2. The 13C-NMR spectrum of compound O shows signals at d 150.12, 106.43, 35.44, 28.36, and 26.36. Deduce the structural formula of compound O.arrow_forwardPropose a structure given the 1H and 13C NMR spectra of the unknown compound. Assign chemical shifts to corresponding hydrogen and carbon atoms Molecular Formula: C5H10O3arrow_forwardA compound reacts with methylmagnesium bromide followed by acidification to form the product with the following 1H NMR spectrum. Identify the compound.arrow_forward
- Compound 1 has molecular formula C7H15Cl. It shows two signals in the 1H-NMR spectrum, one at 1.08 ppm and one at 1.59 ppm. The relative integrals of these two signals are 3 and 2, respectively. Propose structures for compound 1, explaining how you reach your conclusion.arrow_forwardAnalyse the high resolution proton NMR spectrum of a compound with a molecular formula of C8H16O2 and and write its name. Options: A. 2-ethylhexanoic acid B. 1,4-cyclohexanedimethanol C. ethyl hexanoate D. butyl butyrate E. ethyl 2,2-dimethylpropanoatearrow_forwardThe H1H1 NMR spectrum shown corresponds to an unknown compound with the molecular formula C6H10C6H10. There are no strong IR bands between 2100 and 2300 or 3250 and 3350 cm−1. Deduce and draw the structure of the molecule that corresponds to the spectrum.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY