
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2.1, Problem 2.6P
Identify the elements used in each example of molecular art.
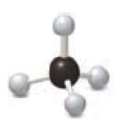
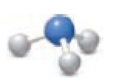
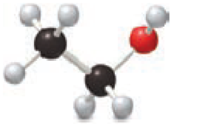
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the table below, there are descriptions of an experiment on samples of three different chemical elements. Decide whether the element is a metal or
nonmetal, if you can. If there is not enough information to decide, choose can't decide in the third column.
element
description
metal or nonmetal?
?
Element 1 is a moderately soft silvery-gray solid. A 5 cm x 5 cm square
of it, only 1 mm thick, is twisted using two pairs of pliers. The sheet
breaks in the middle. The freshly broken edges are lighter colored than
the surface.
metal
1
nonmetal
(can't decide)
Element 2 is a shiny silvery-gray solid. A 10. g cube of it is set on a hot
plate. After 10 minutes, the temperature of the top of the cube has risen
by less than 1 °C.
metal
2
nonmetal
(can't decide)
Element 3 is a hard silvery-gray solid. Wires are fastened to each side of
a 2 cm slab of it, and an ordinary household 9 V battery is hooked up so
that it can feed electricity through the slab to an LED. The LED glows
brightly.
metal…
Two or more substances in variable proportions, where the composition is constant throughout are
a compound.
an element.
a heterogeneous mixture.
a homogeneous mixture.
a crystalline solid.
List the main characteristics that an Earth material must possess to be considered a mineral and describe each.
Compare and contrast the three primary particles contained in atoms.
Distinguish among ionic bonds, covalent bonds, and metallic bonds.
Chapter 2 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Prob. 2.2PCh. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used in each example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - Prob. 2.10P
Ch. 2.2 - Prob. 2.11PCh. 2.2 - Prob. 2.12PCh. 2.2 - Prob. 2.13PCh. 2.3 - Prob. 2.14PCh. 2.3 - Prob. 2.15PCh. 2.3 - Prob. 2.16PCh. 2.3 - Prob. 2.17PCh. 2.3 - Prob. 2.18PCh. 2.4 - Prob. 2.19PCh. 2.4 - Give the period and group number for each element:...Ch. 2.4 - Prob. 2.21PCh. 2.4 - Prob. 2.22PCh. 2.5 - Prob. 2.23PCh. 2.6 - Prob. 2.24PCh. 2.6 - Prob. 2.25PCh. 2.6 - Prob. 2.26PCh. 2.7 - Identify the total number of electrons, the number...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Prob. 2.29PCh. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Which element in each pair has the higher...Ch. 2.8 - Prob. 2.32PCh. 2 - Identify the elements used in each example of...Ch. 2 - Prob. 2.34UKCCh. 2 - Prob. 2.35UKCCh. 2 - Prob. 2.36UKCCh. 2 - Prob. 2.37UKCCh. 2 - Prob. 2.38UKCCh. 2 - Prob. 2.39UKCCh. 2 - Prob. 2.40UKCCh. 2 - Prob. 2.41UKCCh. 2 - Prob. 2.42UKCCh. 2 - Prob. 2.43UKCCh. 2 - Prob. 2.44UKCCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - Prob. 2.47APCh. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - Prob. 2.56APCh. 2 - Prob. 2.57APCh. 2 - Prob. 2.58APCh. 2 - The most common isotope of oxygen has a mass...Ch. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Prob. 2.72APCh. 2 - Prob. 2.73APCh. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Prob. 2.79APCh. 2 - Prob. 2.80APCh. 2 - Prob. 2.81APCh. 2 - Prob. 2.82APCh. 2 - Prob. 2.83APCh. 2 - Arrange the elements in each group in order of...Ch. 2 - Prob. 2.85APCh. 2 - Prob. 2.86APCh. 2 - Answer the following questions about...Ch. 2 - Prob. 2.88APCh. 2 - Prob. 2.89APCh. 2 - (a) What is the chemical formula for...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.93BTCCh. 2 - Prob. 2.95BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name and give symbols for three transition metals in the fourth period. Look up each of your choices in a dictionary, a book such as The Handbook of Chemistry and Physics, or on the Internet, and make a list of their properties. Also list the uses of each element.arrow_forwardA material is believed to be a compound. Suppose you have several samples of this material obtained from various places around the world. Comment on what you would expect to find upon observing the melting point and color for each sample. What would you expect to find upon determining the elemental composition for each sample?arrow_forwardClassify each of these as an element, a compound, a heterogeneous mixture, or a homogeneous mixture. Explain your choice in each case. (a) Chunky peanut butter (b) Distilled water (c) Platinum (d) Airarrow_forward
- A materials engineer has filed for a patent for a new alloy to be used in golf club heads. The composition by mass ranges from 25 to 31% manganese, 6.3 to 7.8% aluminum, 0.65 to 0.85% carbon, and 5.5 to 9.0% chromium, with the remainder being iron. What are the maximum and minimum percentages of iron possible in this alloy? Use Figure 2.12 to snake a prediction about how the density of this alloy would compare with that of iron; justify your prediction.arrow_forwardWhich part of the description of a compound or element refers to its physical properties and which to its chemical properties? (a) The colorless liquid ethanol bums in air. (b) The shiny metal aluminum reacts readily with orange-red brominearrow_forwardClassify each of the following observations about the behavior of a substance as a physical property or chemical property of the substance. a. does not tarnish when exposed to dry air at 25C b. does tarnish when exposed to moist air at 25C c. is a liquid at 75C d. is a solid at 15Carrow_forward
- Please use this word bank to fill in the blanks of the first 5 questions. Atom 1. S is a/an 2. Sg is a/an (Yes! You can use more than one per blank spot!) element molecule Homogenous mixture 3. MgSO4 is a/an 4. A sample of filtered seawater containing Li+, Na+, K+, SO42, NO3¹, and Cl is a/an 5. A grey sample of basalt riddled with golden cubes of pyrite (FeS₂) is a/an 6. What is the state of S8 (s) at room temperature? compound Heterogeneous mixture 7. Give the full atomic symbol of S. 8. Give the subatomic particles of S.arrow_forwardwhat is organic,inorganic, and physical chemistry? give 10 differences with your own wordsarrow_forwardDuring a filtration or distillation experiment, we separate a mixture into its individual components. Do the chemical identities of the components of the mixture change during such a process? Explain.arrow_forward
- which method was the best to see the minutiae?arrow_forwardA substance composed of 2 or more elements in a fixed, definite proportion is considered a homogeneous mixture. a heterogeneous mixture. a molecular compound an alloy. a solution.arrow_forwardWhich statement is true about the elements present in a compound? They maintain their chemical properties. They are composed of many mixtures. They can be separated by physical methods. or They can only be separated by chemical methods.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY