
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 41E
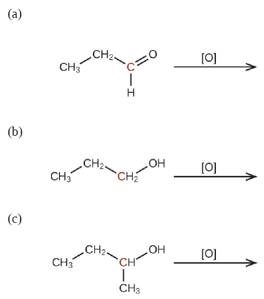
Predict the products of oxidizing the molecules shown in this problem. In each case, identify the product that will result from the minimal increase in oxidation state for the highlighted carbon atom:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the products of reducing the following molecules. In each case, identify the product that will result from the minimal decrease in oxidation state for the highlighted carbon atom:
Give the structural formulas of the compound in the following problem. Explain how you arrived at each answer
When an alkane reacts with an element from group 7A the reaction is referred to as _____. Please explain why.
a) oxidation
b)halogenation
c) combustion
d)displacement
e) decomposition
Chapter 21 Solutions
Chemistry Atoms First2e
Ch. 21 - Write the chemical formula and Lewis structure of...Ch. 21 - What is the difference between the hybridization...Ch. 21 - On a microscopic level, how does the reaction of...Ch. 21 - On a microscopic level, how does the reaction of...Ch. 21 - Explain why unbranched alkenes can form geometric...Ch. 21 - Explain why these two molecules are not isomers:Ch. 21 - Explain why these two molecules are not isomers:Ch. 21 - How does the carbon-atom hybridization change when...Ch. 21 - Write the Lewis structure and molecular formula...Ch. 21 - Write the chemical formula, condensed formula, and...
Ch. 21 - Give the complete IUPAC name for each of the...Ch. 21 - Give the complete IUPAC name for each of the...Ch. 21 - Butane is used as a fuel in disposable lighters....Ch. 21 - Write Lewis structures and name the five...Ch. 21 - Write Lewis structures for the Cis -trans isomers...Ch. 21 - Write structures for the three isomers of the...Ch. 21 - Isooctane is the common name of the isomer of...Ch. 21 - Write Lewis structures and IUPAC names for the...Ch. 21 - Write Lewis structures and IUPAC names for all...Ch. 21 - Name and write the structures of all isomers of...Ch. 21 - Write the structures for all the isomers of the...Ch. 21 - Write Lewis structures and describe the molecular...Ch. 21 - Benzene is one of the compounds used as an octane...Ch. 21 - Teflon is prepared by the polymerization of...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - What mass of 2-bromopropane could be prepared from...Ch. 21 - Acetylene is a very weak acid; however, it will...Ch. 21 - Ethylene can be produced by the pyrolysis of...Ch. 21 - Why do the compounds hexane, hexanol, and hexane...Ch. 21 - Write condensed formulas and provide IUPAC names...Ch. 21 - Give the complete IUPAC name for each of the...Ch. 21 - Give the complete IUPAC name and the common name...Ch. 21 - Write the condensed structures of both isomers...Ch. 21 - Write the condensed structures of all isomers with...Ch. 21 - Draw the condensed formulas for each of the...Ch. 21 - MTBE, Methyl tert -butyl ether, CH3OC(CH3)3, is...Ch. 21 - Write two complete balanced equations for each of...Ch. 21 - Write two complete balanced equations for each of...Ch. 21 - Order the following molecules from least to most...Ch. 21 - Predict the products of oxidizing the molecules...Ch. 21 - Predict the products of reducing the following...Ch. 21 - Explain why it is not possible to possible a...Ch. 21 - How does hybridization of the substituted carbon...Ch. 21 - Fatty acids are carboxylic acids that have long...Ch. 21 - Write a condensed structural formula, such as...Ch. 21 - Write a condensed structural formula, such as...Ch. 21 - The foul odor of rancid butter is caused by...Ch. 21 - Write the two-resonance structures for the acetate...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - Write two complete balanced equations for each of...Ch. 21 - Yields in organic reactions are sometimes low....Ch. 21 - Alcohols A, B and C all have the composition C4H...Ch. 21 - Write the Lewis structures of both isomers with...Ch. 21 - What is the molecular structure about the nitrogen...Ch. 21 - Write the two resonance structures for the...Ch. 21 - Draw Lewis structures for pyridine and its...Ch. 21 - Write the Lewis structures of all isomers with the...Ch. 21 - Write two complete balanced equations for the...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - Identify any carbon atoms that change...Ch. 21 - Identify any carbon atoms that change...Ch. 21 - Identify any carbon atoms that change...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Certain criteria must be satisfied if a measurement or observation is to be believed. Will the criteria necessa...
College Physics
Using the pKa values listed in Table 15.1, predict the products of the following reactions:
Organic Chemistry
A catalyst speeds up a chemical reaction by lowering the activation energy. Sketch Diagram A and draw a second ...
General, Organic, and Biological Chemistry (3rd Edition)
Consider the balanced equation: 2N2H4(g)+N2O4(g)3N2(g)+4H2O(g) Complete the table with the appropriate number o...
Introductory Chemistry (5th Edition) (Standalone Book)
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw and name the eight isomeric alcohols with formula C5H12O.arrow_forwardspecify the geometry of the alkenes [cis, trans, E, Z and NA (not applicable)] for the compounds shown below.arrow_forwardPredict the products of the following reactions. Use structural diagrams and include IUPAC names for all reactants and products. Identify the type of reaction in each case:arrow_forward
- Write the structure of the compound that will be produced in the following reaction? CH3 –C ≡ C–CH2– CH2 – CH3 + 2HBr→arrow_forwardIdentify the hydrocarbon which has the highest oxidation level.arrow_forwardWhat is the range of oxidation states for carbon? Name acompound in which carbon has its highest oxidation state andone in which it has its lowest.arrow_forward
- Identify the possible structural formulae of compound X present in aqueous and basic solutions.arrow_forwarddraw structural formulas for all isomeric alkanes with molecular formula C7H16. Predict which isomer has the lowest boiling point and which has the highest boiling point.arrow_forwardList the compounds that are isomers from lowest to highestarrow_forward
- Show how C4H6O4=C4H4O4 + 2H is an oxidation reactionarrow_forwardConsider the following proposed structures for benzene, each of which is consistent with the molecular formula C6H6. (iv) CH3CCCCCH3 (v) CH2=CHCCH=CH2 When benzene reacts with chlorine to give C6H5Cl, only one isomer of that compound forms. Which of the five proposed structures for benzene are consistent with this observation? When C6H5Cl reacts further with chlorine to give C6H4Cl2, exactly three isomers of the latter compound form. Which of the five proposed structures for benzene are consistent with this observation?arrow_forwardIllustrate the chemical structural formula for 3-methyl-3-ethylpentane. (b) Identify its chemical family as an isomer. (c) Provide the balanced chemical reaction equation for the combustion of one mole of this fuel with an equivalence ratio of ϕ=0.735arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

