
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 10PP
Interpretation Introduction
Interpretation:
The use of periodic acid as a distinguishing reagent between an aldohexose and a ketohexose. Further, the product formed with each of the compounds and the respective number of molar equivalents of
Concept Introduction:
▸ The aldose or ketose carbon sugars that have six membered carbon atoms are referred to as aldohexoses and ketohexoses, respectively. Glucose is an example of aldohexose and fructose is an example of ketohexose. The structures of each have been described below:
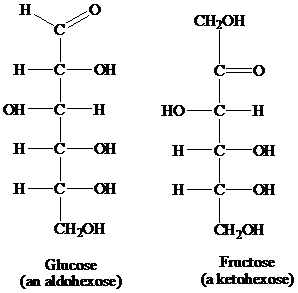
▸ Furthermore, both the aldohexoses and ketohexoses undergo periodate oxidation, in order to yield different products, as a result of the cleavage of the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ribose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in
principle, could contain either a four-membered, five-membered, or six-membered ring.
When D-ribose is treated with methanol in the presence of an acid catalyst, two cyclic
acetals, A and B, are formed, both with molecular formula C,H,0, These are separated,
and each is treated with sodium periodate (Section 10.8C) followed by dilute aqueous
acid. Both A and B yield the same three products in the same ratios.
он о
CHO
СНО
H+ CH,OH A +B
ÕH
1. NalO,
2. H,0*
НО
CHO + CHOH + CH,OH
ÕH
CH,OH
Isomeric cyclic
acetals with molecular
formula CH12O,
D-Ribose
(C;H1605)
From this information, deduce whether the cyclic hemiacetal formed by D-ribose is four-
membered, five-membered, or six-membered.
a) The D-aldopentose A, C5H1005, reacts with HNO3 to yield an optically active aldaric acid B. Kiliani-Fischer
chain extension of A produces a pair of D-aldohexoses C and D. C is converted by HNO3 to an optically active
aldaric acid, but D is converted by HNO3 to an optically inactive aldaric acid. Write acyclic Fischer projections
for A, B, C, D.
b) Disaccharide E is a reducing sugar. It is hydrolyzed by an α-glycosidase enzyme, which means it contains an α-
glycoside link. Treatment of E with Ag2O and excess Mel gives an octamethyl derivative F. Hydrolysis of F in
dilute aqueous acid gives the pair of molecules shown below. Write the structures of E and F. (If the
stereochemistry at a particular carbon is not determined by the above data, indicate this with a wavy line as
shown below.)
HO OMe
OMe
MeO
MeO
MOH
OMe
mOH
OMe
OMe
a) The D-aldopentose A, C5H1005, reacts with HNO3 to yield an optically active aldaric acid B. Kiliani-Fischer
chain extension of A produces a pair of D-aldohexoses C and D. C is converted by HNO3 to an optically active
aldaric acid, but D is converted by HNO3 to an optically inactive aldaric acid. Write acyclic Fischer projections
for A, B, C, D.
Chapter 22 Solutions
Organic Chemistry
Ch. 22 - Prob. 1PPCh. 22 - Prob. 2PPCh. 22 - Prob. 3PPCh. 22 - Prob. 4PPCh. 22 - Prob. 5PPCh. 22 - Prob. 6PPCh. 22 - Prob. 7PPCh. 22 - Prob. 8PPCh. 22 - Practice Problem 22.9 What products would you...Ch. 22 - Prob. 10PP
Ch. 22 - Prob. 11PPCh. 22 - Prob. 12PPCh. 22 - Prob. 13PPCh. 22 - Prob. 14PPCh. 22 - Prob. 15PPCh. 22 - Prob. 16PPCh. 22 - Prob. 17PPCh. 22 - Prob. 18PPCh. 22 - Prob. 19PPCh. 22 - Prob. 20PCh. 22 - Prob. 21PCh. 22 - Prob. 22PCh. 22 - Prob. 23PCh. 22 - Prob. 24PCh. 22 - Prob. 25PCh. 22 - Prob. 26PCh. 22 - Prob. 27PCh. 22 - Prob. 28PCh. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Prob. 32PCh. 22 - Prob. 33PCh. 22 - Prob. 34PCh. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - Prob. 37PCh. 22 - Prob. 38PCh. 22 - Arbutin, a compound that can be isolated from the...Ch. 22 - Prob. 40PCh. 22 - Prob. 41PCh. 22 - Prob. 42PCh. 22 - Prob. 43PCh. 22 - 22.44 The following reaction sequence represents...Ch. 22 - 22.45
The NMR data for the two anomers...Ch. 22 - Shikimic acid is a key biosynthetic intermediate...Ch. 22 - Prob. 1QCh. 22 - Prob. 2QCh. 22 - Give the structural formula of the monosaccharide...Ch. 22 - Prob. 4QCh. 22 - Prob. 5QCh. 22 - Prob. 6QCh. 22 - Prob. 7QCh. 22 - Prob. 8QCh. 22 - Select the reagent needed to perform the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- can you please add an explanation.arrow_forward(d) Draw the structure of the expected product when monosaccharide B undergo mutarotation upon dissolving in water in the presence of Tollens reagent (AGNO3, NHẠOH). он OH O. OH OH OH monosaccharide Barrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed tosynthesize the drug?arrow_forward
- d-Xylose and d-lyxose are formed when d-threose undergoes a Kiliani–Fischer synthesis. d-Xylose is oxidized to an optically inactive aldaric acid,whereas d-lyxose forms an optically active aldaric acid. What are the structures of d-xylose and d-lyxose?arrow_forwardKojibiose is a reducing sugar that forms D-glucose on hydrolysis with aqueous acid. Reaction of kojibiose with iodomethane and Ag20 yields an octamethyl derivative, which can be hydrolyzed with aqueous acid to give one equivalent of 2,3,4,6-tetra-O- methyl-D-glucopyranose and one equivalent of 3,4,6-tri-O-methyl-D-glucopyranose. If kojibiose is hydrolyzed by alpha-glucosidases but not beta-glucosidases, what is its structure?arrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed to synthesize the drug?arrow_forward
- Quinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed to synthesize the drug?arrow_forwardWhat is the product of the starting material D-glyceraldehyde which will (1) produce aldaric acid upon reacting with HNO3 + H2O, NaOCH2, NH2OH, and (CH3CO)2O + NaOCOCH3 (2) produce tartaric acid upon reacting with HNO3 + H2O, NaOCH3, NH2OH, and (CH3CO)2O + NaOCOCH3arrow_forwardDraw the organic products formed in the following reaction.arrow_forward
- The following observations are obtained after a D-hexose was made to react with several reagents: (1) The reactions of a D-hexose with (a) to (d) below yields an aldaric acid (a) NH₂OH, (b) (CH3CO)₂O, NaOCOCH 3, and, (c) NaOCH3, and then, (d) HNO3, H₂O (2) HNO3 oxidation of the same D-hexose gives an aldaric acid. Predict the structures of the three (3) possible hexoses that can undergo the above reactions?arrow_forwardEtoposide, sold as a phosphate derivative with the trade name of Etopophos, is used for the treatment of lung cancer, testicular cancer, and lymphomas. (a) Locate the acetals in etoposide. (b) What products are formed when all of the acetals are hydrolyzed with aqueous acid?arrow_forwardA D-aldopentose A is reduced to an optically active alditol. Upon Kiliani–Fischer synthesis, A is converted to two D-aldohexoses, B and C. B is oxidized to an optically inactive aldaric acid. C is oxidized to an optically active aldaric acid. What are the structures of A–C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning