
Concept explainers
(a)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an
To determine: The percent by mass of M in
(a)
Answer to Problem 173IP
Answer
The mass percent of M in
Explanation of Solution
Explanation
Given
Electronic configuration of
So,
In periodic table atomic number is found to be of the element Zinc.
The molar mass of Zinc is
The molar mass of compound
The molar mass of carbon is
The molar mass of hydrogen is
The molar mass of Zinc is
The molar mass of Bromine is
The molar mass of compound
Therefore the total molar mass of compound
The mass percent of M in
Substitute the value of the molar mass of M and total molar mass of compound, to calculate percent by mass of M in
Therefore, the mass percent of M in
Conclusion
The mass percent of M in
(b)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an organometallic compound which is prepared by treating magnesium or metal atom with a strong acid. The Grignard reaction is defined as an organometallic reaction in which the vinyl, alkyl and aryl-metal halides were added to a carbonyl carbon atom and form an aldehyde or ketone.
To determine: The hybridization of the starred carbon atom in the given figure changed from reactants to products.
(b)
Answer to Problem 173IP
Answer
The hybridization of the starred carbon atom shown in Figure 1 is changed from
Explanation of Solution
Explanation
The given reaction is,
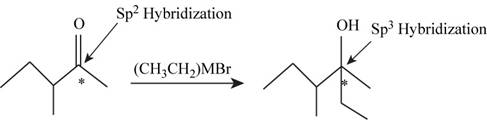
Figure 1
In the given reaction the reactant contains carbonyl group having double bonded oxygen atom hence, the hybridization of the starred carbon in the reactant is
Conclusion
The hybridization of the starred carbon atom shown in Figure 1 is changed from
(c)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an organometallic compound which is prepared by treating magnesium or metal atom with a strong acid. The Grignard reaction is defined as an organometallic reaction in which the vinyl, alkyl and aryl-metal halides were added to a carbonyl carbon atom and form an aldehyde or ketone.
To determine: The systematic name of the product.
(c)
Answer to Problem 173IP
Answer
The systematic name of the product is
Explanation of Solution
Explanation
The given compound is,
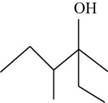
Figure 2
The longest carbon chain contains six carbon atoms numbered from the nearest functional group with an alcohol group present on third carbon. Hence, the root name of the compound is hexanol. It also contains two methyl groups present on third and fourth carbon atom respectively. Hence, the name of the compound is
Conclusion
The systematic name of the product shown in Figure 2 is
Want to see more full solutions like this?
Chapter 22 Solutions
Chemistry
- Alcohols are very useful starting materials for the production of many different compounds. The following conversions, starting with 1-butanol, can be carried out in two or more steps. Show the steps (reactants/catalysts) you would follow to carry out the conversions, drawing the formula for the organic product in each step. For each step, a major product must be produced. (See Exercise 62.) (Hint: In the presence of H+, an alcohol is converted into an alkene and water. This is the exact reverse of the reaction of adding water to an alkene to form an alcohol.) a. 1-butanol butane b. 1-butanol 2-butanonearrow_forwardWhat functional group distinguishes each of the following hydrocarbon derivatives? a. halohydrocarbons b. alcohols c. ethers d. aldehydes e. ketones f. carboxylic acids g. esters h. amines Give examples of each functional group. What prefix or suffix is used to name each functional group? What are the bond angles in each? Describe the bonding in each functional group. What is the difference between a primary, secondary, and tertiary alcohol? For the functional groups in ah, when is a number required to indicate the position of the functional group? Carboxylic acids are often written as RCOOH. What does COOH indicate and what does R indicate? Aldehydes are sometimes written as RCHO. What does CHO indicate?arrow_forwardWhat is a hydrocarbon? What is the difference between a saturated hydrocarbon and an unsaturated hydrocarbon? Distinguish between normal and branched hydrocarbons. What is an alkane? What is a cyclic alkane? What are the two general formulas for alkanes? What is the hybridization of carbon atoms in alkanes? What are the bond angles in alkanes? Why are cyclopropane and cyclobutane so reactive? The normal (unbranched) hydrocarbons are often referred to as straight-chain hydrocarbons. What does this name refer to? Does it mean that the carbon atoms in a straight-chain hydrocarbon really have a linear arrangement? Explain. In the shorthand notation for cyclic alkanes, the hydrogens are usually omitted. How do you determine the number of hydrogens bonded to each carbon in a ring structure?arrow_forward
- . With very reactive agents, such as the halogen elements, alkanes undergo reactions, whereby a new atom rep laces one or more hydrogen atoms of the alkane.arrow_forwarda. Use bond energies (see Table 3-3) to estimate H for the reaction of two molecules of glycine to form a peptide linkage. b. Would you predict S to favor the formation of peptide linkages between two molecules of glycine? c. Would you predict the formation of proteins to be a spontaneous process?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





