
Concept explainers
(a)
Interpretation:
The principal organic product expected when isobutyraldehyde reacts with the lithium enolate of acetone followed by
Concept introduction:
The nucleophilic addition reactions of carbonyl compounds are well known due to the polarity of the carbonyl group. The nucleophile attacks on the carbonyl and adds to the carbonyl carbon. The addition of enolate ion on the carbonyl compounds is known as aldol reaction.
Answer to Problem 22.57AP
The principal organic product obtained when isobutyraldehyde reacts with the lithium enolate of acetone followed by
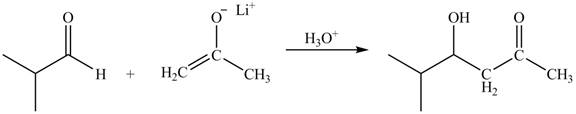
Explanation of Solution
The principal organic product obtained when isobutyraldehyde reacts with the lithium enolate of acetone followed by
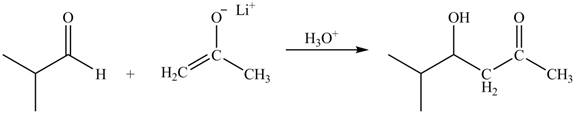
Figure 1
The enolate ions attack rapidly on the carbonyl group. The enolate ion attacks the carbonyl carbon from the carbon-side and undergoes addition on the carbonyl compound.
Lithium enolate of acetone attacks on the isobutyraldehyde from the carbon side and adds on the molecule. The acidic workup converts the oxide ion generated into alcohol group.
The principal organic product obtained when isobutyraldehyde reacts with the lithium enolate of acetone followed by
(b)
Interpretation:
The principal organic product expected when isobutyraldehyde reacts with the lithium enolate of ethyl
Concept introduction:
The nucleophilic addition reactions of carbonyl compounds are well known due to the polarity of the carbonyl group. The nucleophile attacks on the carbonyl and adds to the carbonyl carbon. The addition of enolate ion on the carbonyl compounds is known as aldol reaction.
Answer to Problem 22.57AP
The principal organic product obtained when isobutyraldehyde reacts with the lithium enolate of ethyl
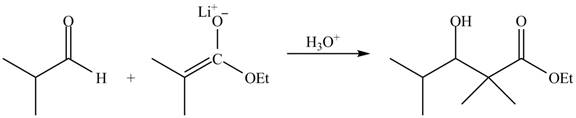
Explanation of Solution
The principal organic product obtained when isobutyraldehyde reacts with the lithium enolate of ethyl

Figure 2
The enolate ions attack rapidly on the carbonyl group. The enolate ion attacks the carbonyl carbon from the carbon-side and undergoes addition on the carbonyl compound.
Lithium enolate of
The principal organic product obtained when isobutyraldehyde reacts with the lithium enolate of ethyl
(c)
Interpretation:
The principal organic product expected when isobutyraldehyde reacts with ethyl
Concept introduction:
The nucleophilic addition reactions of carbonyl compounds are well known due to the polarity of the carbonyl group. The nucleophile attacks on the carbonyl and adds to the carbonyl carbon. The addition of enolate ion on the carbonyl compounds is known as aldol reaction.
Answer to Problem 22.57AP
The principal organic product obtained when isobutyraldehyde reacts with ethyl

Explanation of Solution
The principal organic product obtained when isobutyraldehyde reacts with ethyl

Figure 3
The enolate ions attack rapidly on the carbonyl group. The enolate ion attacks the carbonyl carbon from the carbon-side and undergoes addition on the carbonyl compound.
The zinc metal converts the ethyl
The enolate ion generated attacks on the isobutyraldehyde from the carbon side and adds on the molecule. The acidic workup converts the oxide ion generated into alcohol group.
This reaction is a name reaction known as Reformatsky reaction.
The principal organic product obtained when isobutyraldehyde reacts with ethyl
(d)
Interpretation:
The principal organic product expected when isobutyraldehyde reacts with diethyl malonate and a secondary
Concept introduction:
The nucleophilic addition reactions of carbonyl compounds are well known due to the polarity of the carbonyl group. The nucleophile attacks on the carbonyl and adds to the carbonyl carbon. The addition of enolate ion on the carbonyl compounds is known as aldol reaction.
Answer to Problem 22.57AP
The principal organic product obtained when isobutyraldehyde reacts with diethyl malonate and a secondary amine
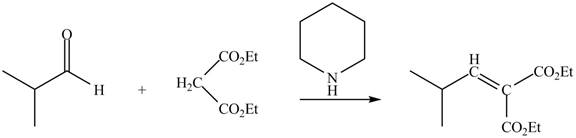
Explanation of Solution
The principal organic product obtained when isobutyraldehyde reacts with diethyl malonate and a secondary amine
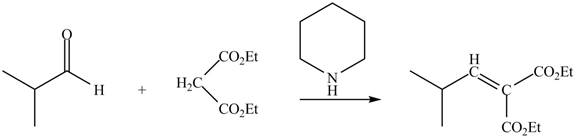
Figure 4
The enolate ions attack rapidly on the carbonyl group. The enolate ion attacks the carbonyl carbon from the carbon-side and undergoes addition on the carbonyl compound.
Malonic ester is converted into the enolate ion by the secondary amine taken as the catalyst. The secondary amine pyridine taken here is basic in nature and takes up the acidic proton of the malonic ester.
The enolate ion generated attacks on the isobutyraldehyde from the carbon side and adds on the molecule. The acidic workup converts the oxide ion generated into alcohol group.
This reaction is a name reaction known as Knoevenagal reaction.
The principal organic product obtained when isobutyraldehyde reacts with diethyl malonate and a secondary amine
(e)
Interpretation:
The principal organic product expected when isobutyraldehyde reacts with ethyl acetoacetate and a secondary amine
Concept introduction:
The nucleophilic addition reactions of carbonyl compounds are well known due to the polarity of the carbonyl group. The nucleophile attacks on the carbonyl and adds to the carbonyl carbon. The addition of enolate ion on the carbonyl compounds is known as aldol reaction.
Answer to Problem 22.57AP
The principal organic product obtained when isobutyraldehyde reacts with ethyl acetoacetate and a secondary amine
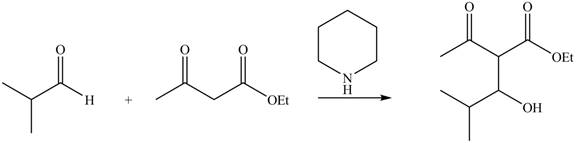
Explanation of Solution
The principal organic product obtained when isobutyraldehyde reacts with ethyl acetoacetate and a secondary amine

Figure 5
The enolate ions attack rapidly on the carbonyl group. The enolate ion attacks the carbonyl carbon from the carbon-side and undergoes addition on the carbonyl compound.
Ethyl acetoacetate is converted into the enolate ion by the secondary amine taken as the catalyst. The secondary amine pyridine taken here is basic in nature and takes up the acidic proton of the malonic ester.
The enolate ion generated attacks on the isobutyraldehyde from the carbon side and adds on the molecule. The acidic workup converts the oxide ion generated into alcohol group.
This reaction is a name reaction known as Knoevenagal reaction.
The principal organic product obtained when isobutyraldehyde reacts with ethyl acetoacetate and a secondary amine
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Following is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardThe base-promoted rearrangement of an a-haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. Jo Han Sow La NaOH, THF O Nat H3O+ OH NaOH, THF The mechanism involves the following 5 steps: -ō cyclopropanone intermediate 1. Abstraction of a proton to form enolate anion 1; 2. Formation of a cyclopropanone intermediate 2 with expulsion of chloride ion; 3. Addition of hydroxide ion to form tetrahedral intermediate 3; 4. Collapse of the tetrahedral intermediate and breakage of the three-membered ring to form carbanion intermediate 4; 5. Proton transfer to form the rearranged carboxylic acid. For the following reaction, draw the reaction out on paper, and then draw the structure of cyclopropanone intermediate 2 in the window. 1. NaOH, THF 2. H3O* OHarrow_forward
- Acid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardWhen cis-2-decalone is dissolved in ether containing a trace of HCl, an equilibrium is established with trans-2-decalone. The latter ketone predominates in the equilibrium mixture. Propose a mechanism for this isomerization and account for the fact that the trans isomer predominates at equilibrium.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forward
- One step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.arrow_forwardFollowing is a retrosynthetic analysis for an intermediate in the industrial synthesis of vitamin A. (a) Addition of one mole of HCl to isoprene gives 4-chloro-2-methyl-2-butene as the major product. Propose a mechanism for this addition and account for its regioselectivity. (b) Propose a synthesis of the vitamin A precursor from this allylic chloride and ethyl acetoacetate.arrow_forwardWhen cis-4-chlorocyclohexanol is treated with sodium hydroxide in ethanol, it gives mainly the substitution product trans-1,4-cyclohexanediol (1). Under the same reaction conditions, trans-4-chlorocyclohexanol gives 3-cyclohexenol (2) and the bicyclic ether (3). (a) Propose a mechanism for formation of product (1), and account for its configuration. (b) Propose a mechanism for formation of product (2). (c) Account for the fact that the bicyclic ether (3) is formed from the trans isomer but not from the cis isomer.arrow_forward
- Treatment of an aldehyde or ketone with cyanide ion (-:C=N), followed by protonation of the tetrahedral alkoxide ion intermediate, gives a cyanohydrin. Show the structure of the cyanohydrin obtained from cyclohexanone.arrow_forwardThe tosylate of a primary alcohol normally undergoes an SN2 reaction with hydroxide ion to give a primary alcohol. Reaction of this tosylate, however, gives a compound of molecular formula C7H12O. Propose a structural formula for this compound and a mechanism for its formation.arrow_forwardDihydropyran is synthesized by treating tetrahydrofurfuryl alcohol with an arenesulfonic acid, ArSO3H. Propose a mechanism for this conversion.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


