
Concept explainers
(a)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
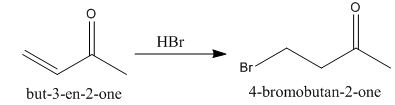
Explanation of Solution
The principal organic product obtained when

Figure 1
In this reaction, the addition of
The principal organic product obtained when
(b)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
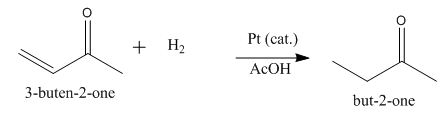
Explanation of Solution
The principal organic product obtained when

Figure 2
In this reaction, the addition of
The principal organic product obtained when
(c)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
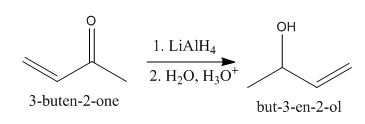
Explanation of Solution
The principal organic product obtained when

Figure 3
In this reaction, the addition of
The principal organic product obtained when
(d)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
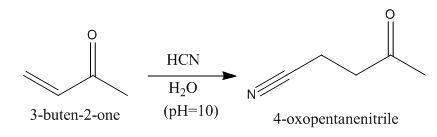
Explanation of Solution
The principal organic product obtained when

Figure 4
In this reaction, the addition of
In the product, hydrogen will add to that carbon of the double bond that has the least number of hydrogens.
The principal organic product obtained when
(e)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
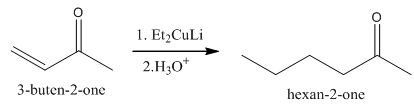
Explanation of Solution
The principal organic product obtained when

Figure 5
The reaction of
The principal organic product obtained when
(f)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
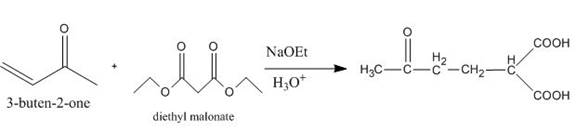
Explanation of Solution
The principal organic product obtained when

Figure 6
The reaction of
The principal organic product obtained when
(g)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
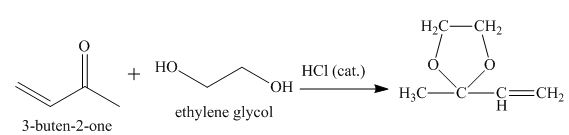
Explanation of Solution
The principal organic product obtained when

Figure 7
The reaction of
The principal organic product obtained when
(h)
Interpretation:
The principal organic product expected when
Concept introduction:
Diels-Alder reaction is a cycloaddition reaction. The reaction is known as a
Answer to Problem 22.55AP
The principal organic product obtained when
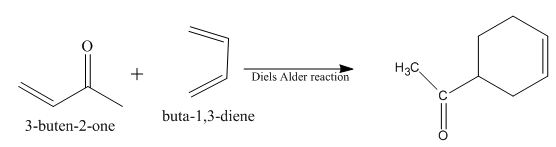
Explanation of Solution
The principal organic product obtained when

Figure 8
The reaction of
The principal organic product obtained when
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- When A is reacted with hot aqueous NaOH, a compound B of molecular formula C8H11NO is produced. With this information, write the correct structure of B and propose the reaction mechanism (step by step, with the correct use of arrows) to obtain B.arrow_forwardBenzoic acid, Ph-COOH (C6H5CO2H), is not soluble in water while it dissolves in ether (diethyl ether), (CH3CH2)2O. Yet upon treatment with sodium hydroxide, benzoic acid turns hydrophilic and dissolves in water. Provide chemical explanation of this observation.arrow_forwardGive a proposal on the synthesis methods of CoSO4.7H2Oarrow_forward
- 5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forward3. The reaction of bromine with 3-hexanone produces two isomeric products of formula C6H11BrO. Give the mechanism for obtaining the two isomers.arrow_forwardA solution of acetone [(CH3)2C=O] in ethanol (CH3CH2OH) in the presence of a trace of acid was allowed to stand for several days, and a new compound of molecular formula C7H16O2 was formed. The IR spectrum showed only one major peak in the functional group region around 3000 cm−1, and the 1H NMR spectrum is given here. What is the structure of the product?arrow_forward
- Reaction of iodoethane with CN- yields a small amount of isonitrile, CH3CH2N≡C, along with the nitrile CH3CH2N≡N, as the major product. Write electron-dot structures for both products, assign formal charges as necessary, and propose mechanisms to account for their formation.arrow_forwardThree isomeric pentanols with unbranched carbon chains exist. Which of these isomers, upon dehydration at 180C, yields only 1-pentene as a product?arrow_forward3 b and c) Give the productarrow_forward
- Provide the structure of the major organic product of the following reaction and? explain the stereochemistry which results in this product. 2-Pentanol reacting with 1.) PBr3, pyridine 2.) NaCNarrow_forwardthe organic compound 2-heptanone, belonging to the ketone family, is responsible for the strong penetrating odor in Roquefort cheeses. Starting from acetylene as the starting reagent, propose a synthesis line with the reaction mechanisms involved for the synthetic obtaining of 2-heptanone and use it as a food additive in analogous cheeses.arrow_forwardCompound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct. Can be more than one answerarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



