
a)
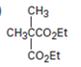
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
The compound shown can be prepared by using malonic ester synthesis.
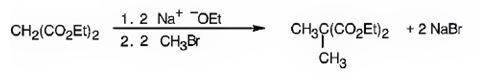
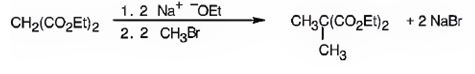
Explanation of Solution
The compound shown is a derivative of carboxylic acid. Hence it can be prepared using malonic ester synthesis. The acid has two methyl groups attached to the carbon adjacent to ester groups. It can be prepared by replacing the two hydrogens on the active methylene group of malonic ester by two methyl groups. This is achieved by treating the ester with two equivalents of sodium ethoxide and two equivalents of methyl bromide.
The compound shown can be prepared by using malonic ester synthesis.
b)
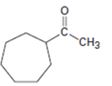
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
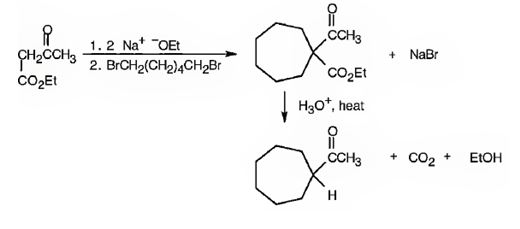
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
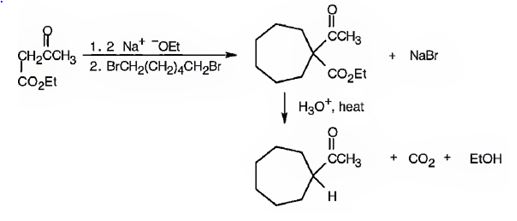
Explanation of Solution
: The compound is a methyl ketone. Hence it can be prepared using aceto acetic ester synthesis. The base ethoxide ion abstracts a proton from the active methylene group of acetoacetic ester to yield the enolate anion. The nucleophilic attack of the anion on 1,6- dibromohexane displaces the bromide ion to produce a α- substituted acetoacetic ester. The second acidic hydrogen of the ester is then removed by another ethoxide ion which is followed by the nucleophilic attack of the anion on the other carbon bearing bromine to produce a cyclic ester. Upon treating with aqueous acids the ester group gets hydrolyzed to give a β- ketocarboxylic acid. The ketocarboxylic acid eliminates a CO2 molecule on heating to yield methyl cycloheptyl ketone.
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
c)
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
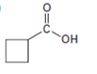
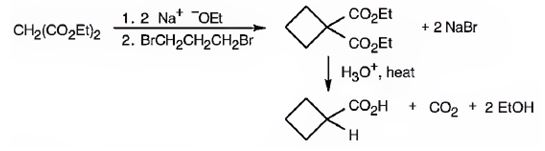
The compound shown can be prepared by using malonic ester synthesis.
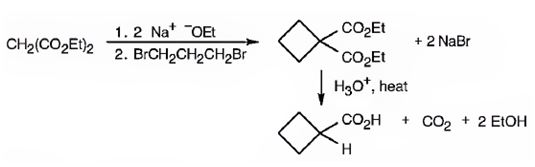
Explanation of Solution
The compound shown is a carboxylic acid. Hence it can be prepared using malonic ester synthesis. The base ethoxide ion abstracts a proton from the active methylene group of malonic ester to yield the enolate anion. The nucleophilic attack of the anion on 1,3- dibromopropane displaces the bromide ion to produce a α- substituted malonic ester. The second acidic hydrogen of the ester is then removed by another ethoxide ion which is followed by the nucleophilic attack of the anion on the other carbon bearing bromine to produce a cyclic diester. Upon treating with aqueous acids the ester groups get hydrolyzed to give a dicarboxylic acid. The dicarboxylic acid eliminates a CO2 molecule on heating to yield cyclobutylcarboxylic acid.
The compound shown can be prepared by using malonic ester synthesis.
d)
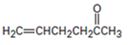
Interpretation:
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis is to be shown.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To show
How to prepare the compound shown using either an acetoacetic ester synthesis or malonic ester synthesis.
Answer to Problem 46AP
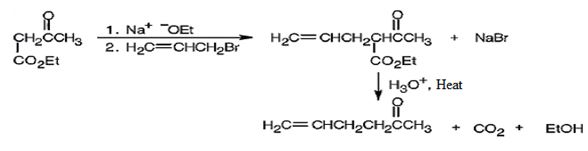
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
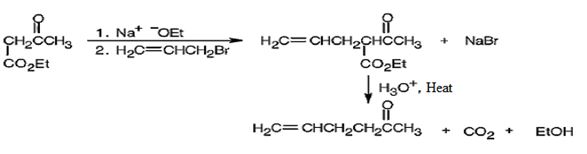
Explanation of Solution
The compound is a methyl ketone. Hence it can be prepared using aceto acetic ester synthesis. The base ethoxide ion abstracts a proton from the active methylene group of acetoacetic ester to yield the enolate anion. The nucleophilic attack of the anion on allyl bromide displaces the bromide ion to produce α- allylsubstituted acetoacetic ester. Upon treating with aqueous acids the ester group gets hydrolyzed to give a β- ketocarboxylic acid. The ketocarboxylic acid eliminates a CO2 molecule on heating to yield hex-5-ene-2-one.
The compound shown can be prepared by using an acetoacetic ester synthesis as shown below.
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- Show how the following compound can be prepared by a malonic or acetoacetic ester synthesis (or other B-dicarbonyl) reaction:arrow_forwardShow how the malonic ester synthesis is used to prepare 2-benzylbutanoic acid.Sarrow_forwardHow could you convert N-methylbenzamide to the following compounds? a. N-methylbenzylamine b. benzoic acid c. methyl benzoate d. benzyl alcoholarrow_forward
- If methanol rather than water is added at the end of a Hell-Volhard-Zelinskii reaction, an ester rather than an acid is produced. Show how you would carry out the following transformation, and propose a mechanism for the ester-forming step.arrow_forwardHow might you prepare the following esters using a nucleophilic acyl substitution reaction of an acid chloride? (a) CH3CH2CO2CH3 (b) CH3CO2CH2CH3 (c) Ethyl benzoatearrow_forwardShow how to synthesize the following compound using either the malonic ester synthesis or the acetoacetic ester synthesis. Q.) Cyclobutyl methyl ketonearrow_forward
- Show how to synthesize the following compound using either the malonic ester synthesis or the acetoacetic ester synthesis. Q.) Cyclopropanecarboxylic acidarrow_forwardHow would you prepare the following estersarrow_forwardShow how the acetoacetic ester synthesis is used to make 3-propylhex-5-en-2-one.Sarrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

