
a)
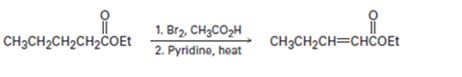
Interpretation:
What is wrong with the synthetic route given is to be stated.
Concept introduction:
To state:
What is wrong with the synthetic route given?
b)
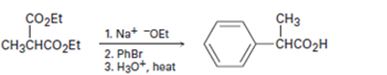
Interpretation:
What is wrong with the synthetic route given is to be stated.
Concept introduction:
Acetoacetic ester when treated with sodium ethoxide yields an enolate anion. The enolate ion will undergo a SN2 displacement reaction with primary
To state:
What is wrong with the synthetic route given?
c)

Interpretation:
What is wrong with the synthetic route given is to be stated.
Concept introduction:
Acetoacetic ester synthesis can be used to prepare methyl ketones. Carboxylic acids can be prepared by malonic ester synthesis.
To state:
What is wrong with the synthetic route shown?
Trending nowThis is a popular solution!

Chapter 22 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

