
(a)
Interpretation:
The product obtained in the reaction of isopropylamine with dilute
Concept introduction:
Answer to Problem 23.46AP
The product
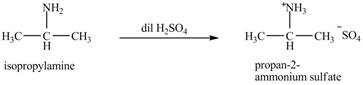
Explanation of Solution
The reaction of amines with dilute

Figure 1
The product obtained in the reaction of isopropylamine with dilute
(b)
Interpretation:
The product obtained in the reaction of isopropylamine with a dilute
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The reaction does not form any product. The reaction is shown below.
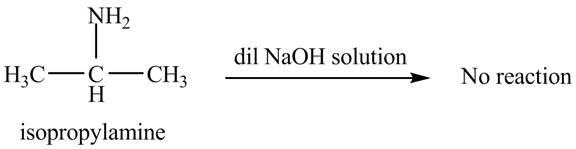
Explanation of Solution
When isopropylamine reacts with a dilute
Figure 2
No product is obtained in the reaction of isopropylamine with a dilute
(c)
Interpretation:
The product obtained in the reaction of isopropylamine with butyllithium in
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The product lithium isopropylamide is obtained in the reaction of isopropylamine with butyllithium in
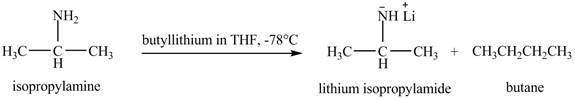
Explanation of Solution
The organolithium base butyllithium in

Figure 3
The product obtained in the reaction of isopropylamine with butyllithium in
(d)
Interpretation:
The product obtained in the reaction of isopropylamine with acetyl chloride and pyridine is to be stated.
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The product,
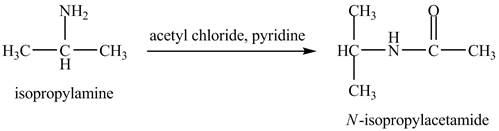
Explanation of Solution
The acid chloride is a very reactive compound. When it reacts with amines it forms amides. The reaction of isopropylamine with acetyl chloride in pyridine results in the formation of
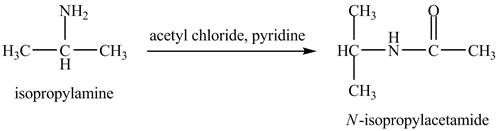
Figure 4
The product obtained in the reaction of isopropylamine with acetyl chloride and pyridine is
(e)
Interpretation:
The product obtained in the reaction of isopropylamine with
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. The formation of diazonium salt from
Answer to Problem 23.46AP
The product,
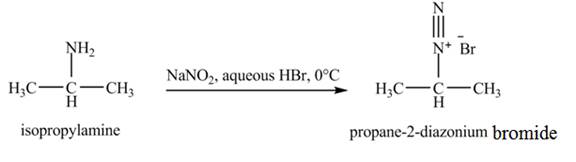
Explanation of Solution
The reaction is an example of a diazotization reaction. When isopropylamine reacts with

Figure 5
The product that obtained in the reaction of isopropylamine with
(f)
Interpretation:
The product obtained in the reaction of isopropylamine with acetone and
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
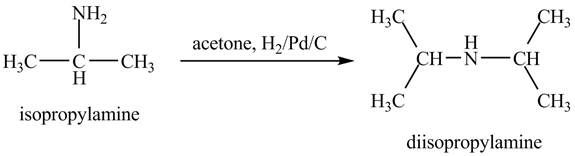
Explanation of Solution
When acetone reacts with isopropylamine, it forms enamine, with double bonds. It is then hydrogenated by

Figure 6
The product obtained in the reaction of isopropylamine with acetone and
(g)
Interpretation:
The product obtained in the reaction of isopropylamine with excess
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Hofmann elimination reaction occurs as an anti-elimination reaction. In this reaction, the starting material is quaternary ammonium hydroxide. When quaternary ammonium hydroxide is heated,
Answer to Problem 23.46AP
The product,
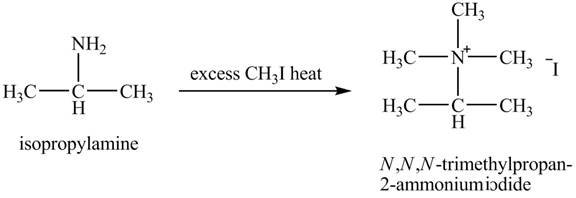
Explanation of Solution
Hofmann elimination reaction occurs as an anti-elimination reaction. In this reaction, the starting material is isopropylamine. When it reacts with an excess of

Figure 7
The product obtained in the reaction of isopropylamine with excess
(h)
Interpretation:
The product obtained in the reaction of isopropylamine with benzoic acid at
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The product
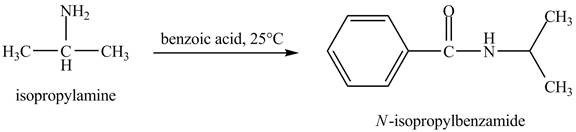
Explanation of Solution
In this reaction, benzoic acid is a reactive compound. When it reacts with amine it results in the formation of an amide. This reaction is similar to the conversion of carboxylic acid to amides. The reaction is shown below.
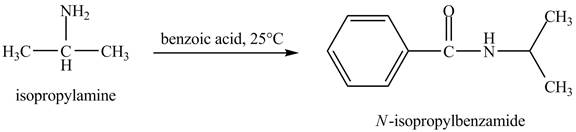
Figure 8
The product obtained in the reaction of isopropylamine with benzoic acid at
(i)
Interpretation:
The product obtained in the reaction of isopropylamine with formaldehyde,
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The product
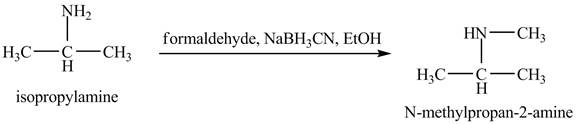
Explanation of Solution
This reaction is an example of a reductive amination reaction. Reductive amination reaction is the conversion of the carbonyl group to the amine or it converts one amine to newer amine. In this reaction, when isopropylamine reacts formaldehyde,

Figure 9
The product that obtained in the reaction of isopropylamine with formaldehyde,
(j)
Interpretation:
The product obtained in the reaction of isopropylamine with
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The product
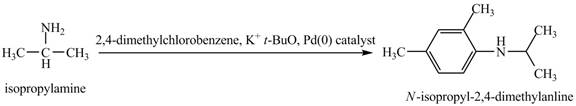
Explanation of Solution
This reaction is an example of a Buchwald-Hartwig reaction. Buchwald Hartwig reaction is used for
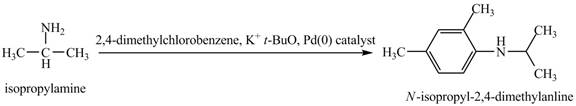
Figure 10
The product obtained in the reaction of isopropylamine with
(k)
Interpretation:
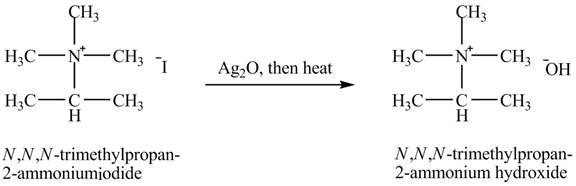
The product obtained in the reaction of isopropylamine with a product of part (g) +
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Hofmann elimination reaction occurs as an anti-elimination reaction. In this reaction, the starting material is quaternary ammonium hydroxide. When quaternary ammonium hydroxide is heated,
Answer to Problem 23.46AP
The product
Explanation of Solution
When isopropylamine undergoes Hofmann elimination reaction with a product of part (g)

Figure 11
The product obtained in the reaction of isopropylamine with a product of part (g)
(l)
Interpretation:
The product obtained in the reaction of isopropylamine with a product of part (d) with
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia with a substituent. It may be alkyl or aryl group. Amines are basic in nature because the nitrogen can donate its lone pairs and it also has the ability to accept the proton in water.
Answer to Problem 23.46AP
The product,
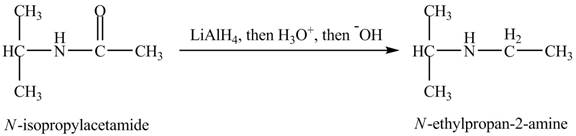
Explanation of Solution
When
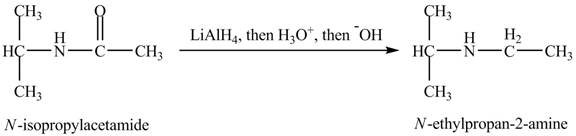
Figure 12
The product obtained in the reaction of isopropylamine with a product of part (d) with
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- If a quaternary ammonium ion can undergo an elimination reaction with a strong base, why can’t a protonated tertiary amine undergo the same reaction?arrow_forward1,4-Diazabicyclo[2.2.2]octane (abbreviated DABCO) is a tertiary amine that catalyzes transesterification reactions. Explain how it does this.arrow_forwardWrite the chemical equation for the acid dissociation of acetaminophen, C8H9O2N. Write the Ka expression for the acid dissociation of acetaminophen.arrow_forward
- Amide hydrolysis in basic conditions forms A. a carboxylic acid and an amine B. a carboxylate salt and an amine 3. an ester and an amine 4. a carboxylic acid and an amine saltarrow_forwardWhich of the following alcohols will give a carboxylic acid product when reacted with K2Cr2O7? 2-Butanol Methanol Tert-butyl-alcohol All of these will be oxidized to carboxylic acids. 2. Aromatic amines are _________ acidic, but stronger than the aliphatic amines are unique as they do not behave like any of the other amines neutral because of the polarity basic, but weaker than aliphatic amines 3. Which of the following is true of the Fischer esterification reactions? It is an equilibrium reaction that can never be driven far to the right. It proceeds until the limiting reagent is completely used up. It is an equilibrium reaction that can be driven to the right by the addition of water. It is an equilibrium reaction that can be driven to the right by the removal of water.arrow_forwardProvide the structural formula of the products that are formed when acetophenone reacts with these reagents: 1. NaBH4 in Methanol 2. Ag(NH3)2+ 3. C6H5MgBr, then H3O+ 4. H2 in Pt 5. Hydroxylamine 6. Phenylhydrazine 7. LiAlH4 then water 8. HCN 9. Diethylamine 10. 2 CH3OH, HCl catalystarrow_forward
- Predict the products and proposemechanisms for the reactions ofcarboxylic acids with reducingagents, alcohols, amines, andorganometallic reagentsarrow_forwardAn amine of unknown structure contains one nitrogen and nine carbon atoms. The 13C-NMR spectrum shows only five signals, all between 20 and 60 ppm. Three cycles of Hofmann elimination sequence [(1) CH3I; (2) Ag2O, H3O; (3) heat] give trimethylamine and 1,4,8-nonatriene. Propose a structural formula for the amine.arrow_forwardPredict the major products formed when the following amines undergo exhaustivemethylation, treatment with Ag2O, and heating.(a) hexan-2-aminearrow_forward
- 3 Compare the basicity of amines with other common bases, and explain how theirbasicity varies with hybridization and aromaticity.arrow_forward(a) Draw the structures of the following compounds :(i) 4-Chloropentan-2-one (ii) p-Nitropropiophenone(b) Give tests to distinguish between the following pairs of compounds :(i) Ethanal and Propanal (ii) Phenol and Benzoic acid(iii) Benzaldehyde and Acetophenonearrow_forward3 Would the product be a(n) a) carbinolamine b) hydrazone c) imine d) hydrazine e) aminalarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



