
Concept explainers
(a)
Interpretation:
The increasing order of basicity of propylamine, ammonia, and dipropylamine in aqueous solution is to be arranged.
Concept introduction:
Answer to Problem 23.7P
The increasing order of basicity of propylamine, ammonia, and dipropylamine in aqueous solution is shown below.
Explanation of Solution
It is known that the order of basicity of amines in aqueous solution is
The increasing order of basicity of propylamine, ammonia, and dipropylamine in aqueous solution is shown below.
(b)
Interpretation:
The increasing order of basicity of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.7P
The increasing order of basicity of
Explanation of Solution
It is known that the order of basicity of amines in aqueous solution solution is
The increasing order of basicity of
(c)
Interpretation:
The increasing order of basicity of aniline, methyl m-aminobenzoate, and methyl p-aminobenzoate in aqueous solution is to be arranged.
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.7P
The increasing order of basicity of aniline, methyl m-aminobenzoate, and methyl p-aminobenzoate in aqueous solution is shown below.
Explanation of Solution
The structure of aniline, methyl m-aminobenzoate, and methyl p-aminobenzoate is shown below.
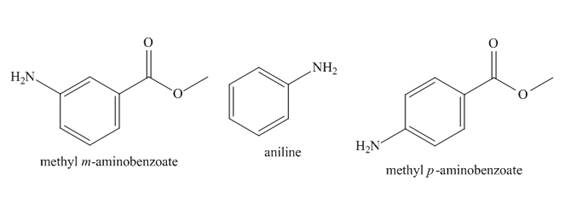
Figure 1
Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
The basicity of aniline substituted compound depends on the group that is attached to benzene ring. If the group attached to benzene ring is electron withdrawing group, it will decrease the basicity of the compound. If the group attached to benzene ring is electron donating group, it will increase the basicity of the compound.
There is no group attached in case of aniline. Therefore, it is most basic. In case of methyl m-aminobenzoate and methyl p-aminobenzoate, the
The increasing order of basicity of aniline, methyl m-aminobenzoate, and methyl p-aminobenzoate in aqueous solution is shown below.
(d)
Interpretation:
The increasing order of basicity of benzylamine, p-nitrobenzylamine, cyclohexylamine, aniline in aqueous solution is to be arranged.
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.7P
The increasing order of basicity of benzylamine, p-nitrobenzylamine, cyclohexylamine, aniline in aqueous solution is shown below.
Explanation of Solution
The structure of benzylamine, p-nitrobenzylamine, cyclohexylamine, aniline is shown below.
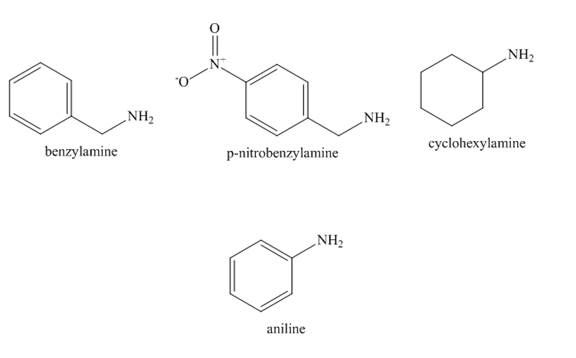
Figure 2
Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
In case of aniline, the lone pair present at nitrogen atom is resonate with the benzene ring. Therefore, the electron density on nitrogen is decreased. Therefore, it is least basic.
In case of cyclohexylamine, there is no resonance. The cyclohexyl group is an electron donating group which increases the electron density on nitrogen atom. Therefore, the basicity of cyclohexylamine increased.
In case of benzylamine there is also no resonance because
In case of p-nitrobenzylamine there is also no resonance because
The increasing order of basicity of benzylamine, p-nitrobenzylamine, cyclohexylamine, aniline in aqueous solution is shown below.
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Phenacetin is an analgesic compound having molecular formula C10H13NO2. Once a common component in over-thecounter pain relievers such as APC (aspirin, phenacetin, caffeine), phenacetin is no longer used because of its liver toxicity. Deduce the structure of phenacetin from its 1H NMR and IR spectra.arrow_forwardAccount for the following :(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).(ii) Aniline does not undergo Friedel – Crafts reaction.(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.arrow_forward3 Compare the basicity of amines with other common bases, and explain how theirbasicity varies with hybridization and aromaticity.arrow_forward
- Histamine, whose release in the body triggers nasal secretions and constricted airways, has three nitrogen atoms. List them in order of increasing basicity and explain your ordering.arrow_forwardGive a suitable chemical reaction to distinguish between a primary and a secondary amine. Suggest one way of Increasing the basicity of an amine and give a specific example of its application.arrow_forwardThe two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides.Show how these techniques can be used to accomplish the following syntheses.(a) benzoic acid S benzylamine (b) benzaldehyde S benzylamine(c) pyrrolidine S N@ethylpyrrolidine (d) cyclohexanone S N@cyclohexylpyrrolidine(e) HOOC¬(CH2)3 ¬COOH S pentane@1,5@diamine (cadaverine)arrow_forward
- Following is an outline of a synthesis of the bronchodilator carbuterol, a beta-2 adrenergic blocker with high selectivity for airway smooth muscle receptors. Q.Suggest a structural relationship between carbuterol and ephedrinearrow_forwardThe following three derivatives of succinimide are anticonvulsants that have found use in the treatment of epilepsy, particularly petit mal seizures. Q. Of these three anticonvulsants, one is considerably more acidic than the other two. Which is the most acidic compound? Estimate its pKa and account for its acidity. How does its acidity compare with that of phenol? with that of acetic acid?arrow_forwardBy discussing the basicities of the amine structures given below, list the basicities from large to small.a) Anilineb) Pyridinec) 4-Methoxyanilined) Diphenylaminee) Piperidinearrow_forward
- Aliphatic amines are more basic than ammonia, whereas aromatic amines are less basic than ammonia. Is that true or false?arrow_forwardArrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. HF, CH3CH2CH2OH, CH3CH2COOH 2. Ethyl amine, Ethanol, Propanearrow_forwardHypoglycin A, an amino acid derivative found in unripened lychee, is anacutely toxic compound that produces seizures, coma, and sometimesdeath in undernourished children when ingested on an empty stomach. (a) Draw the neutral, positively charged, and negatively charged forms of hypoglycin A. (b) Which form predominates at pH = 1, 6, and 11? (c) What is the structure of hypoclycin A at its isoelectric point?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


