
a)
Interpretation:
Which aldehyde or
Concept introduction:
To state:
Which aldehyde or ketone gives in an aldol reaction the compound represented by the model.
Answer to Problem 23VC
The ketone that gives in an aldol reaction the compound represented by the model is 3-pentanone.
Explanation of Solution
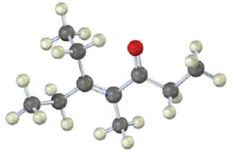
The compound represented by the model is
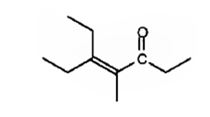
An analysis of the structure indicates it can be produced by the aldol reaction between two 3-pentanone molecules and dehydrating the aldol obtained.
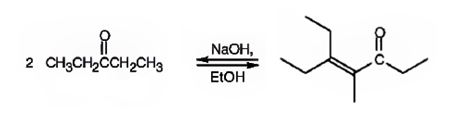
The ketone that gives in an aldol reaction the compound represented by the model is 3-pentanone.
b)
Interpretation:
Which aldehyde or ketone gives in an aldol reaction the compound represented by the model is to be stated.
Concept introduction:
Aldehydes with α-hydrogen undergo a base catalyzed carbonyl condensation reaction in aldol condensation. In this reaction two molecules of the reactant combine by forming a bond between α- carbon of one molecule and the carbonyl carbon of the second molecule. The product obtained is a β-hydroxyaldehyde.
To state:
Which aldehyde or ketone gives in an aldol reaction the compound represented by the model.
Answer to Problem 23VC
The aldehyde that gives in an aldol reaction the compound represented by the model is 3-methylbutanal.
Explanation of Solution
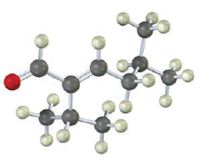
The compound represented by the model is
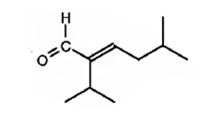
An analysis of the structure indicates it can be produced by the aldol reaction between two 3-methylbutanal molecules and dehydrating the aldol obtained.
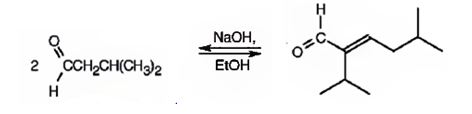
The aldehyde that gives in an aldol reaction, the compound represented by the model is 3-methylbutanal.
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- What vinylic halide couples with styrene (C6H5 - CH = CH2) in order to synthesize each of the following compounds using a Heck reaction?arrow_forwardWhat aldol product is formed when two molecules of butanal react together in the presence of base? What reagents are needed to convert this product to each of the following compounds?arrow_forwardIllustrate the steps of a directed aldol reaction between a ketoneand an aldehyde, both of which have α hydrogens ?arrow_forward
- Acid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardThe following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?arrow_forwardWhat two sets of reagents (each consisting of a carbonyl compound and phosphonium ylide) can be used for the synthesis of the following alkene? a. What alkyl halide is required to prepare each of the phosphonium ylides? b. What is the best set of reagents to use for the synthesis?arrow_forward
- Give the major product if the following reactionsarrow_forwardWhat two sets of reagents (each consisting of a carbonyl compound and phosphonium ylide) can be used for the synthesis of each of the following alkenes? What alkyl halide is required to prepare each of the phosphonium ylides? What is the best set of reagents to use for the synthesis?arrow_forwardFrom what aldehyde or ketone could each of the following be prepared by reduction with NaBH4 or LiAlH4?arrow_forward
- What is the result of treating the following epoxide with acidic solution of H3O+arrow_forwardWhat is the likely mechanism of nucleophilic substitution for each alkyl halide?arrow_forwardDraw structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol or enone below.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

