
Concept explainers
(a)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
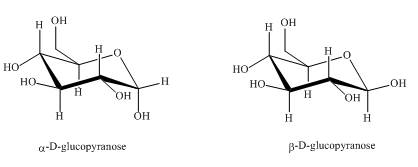
Explanation of Solution
The compounds,
They are diastereomers also as they are not mirror images of each other. The structure of both the compounds is shown below.

Figure 1
These two compounds are anomers as well as diastereomers as shown in Figure 1.
(b)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
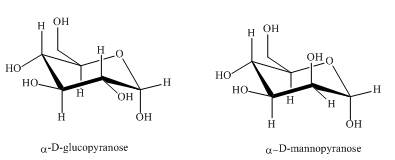
Explanation of Solution
Both the above structures of

Figure 2
The compounds
(c)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
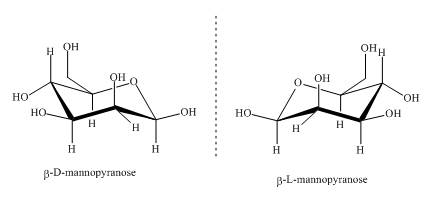
Explanation of Solution
Sugars can be divided into two groups based on the symmetry of carbon atoms. If the molecule has asymmetric carbon atom it will have a non superimposable mirror image. The non superimposable mirror images are known as enantiomers. As the given compounds are non super imposable mirror images of each other they are enantiomers. This can be well explained by the illustrations shown below.

Figure 3
These two compounds
(d)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
The compounds,
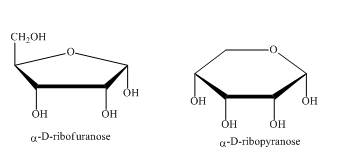
Explanation of Solution
As the isomers are the compounds, having similar chemical formula but different structures. These two compounds have same chemical formula. The chemical structure is of these two compounds is quite different as shown in Figure 4.

Figure 4
In the above shown compounds, one is a pentose sugar furanose while the other is a hexose sugar pyranose.
These two compounds,
(e)
Interpretation:
Whether
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons.
Answer to Problem 24.39AP
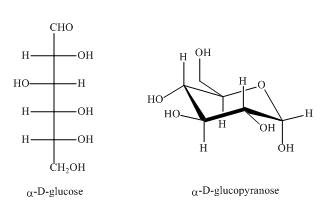
Explanation of Solution
As the isomers are the compounds, having similar chemical formula but different structures. Both the above structures are constitutional isomers as shown below in the Figure.

Figure 5
In the above shown compounds, one is a ring structure having keto group while the other is open ring structure having aldehyde as the functional group. The chemical formula of the given compounds is same but their properties are totally different.
The given compounds
(f)
Interpretation:
Whether the compounds,
Concept introduction:
Sugars show different types of isomerism between molecules. They may be enantiomers, epimers, anomers, or diastereomers depending upon chirality and plane of symmetry in molecules. It also depends on stereochemistry of different carbons. The naming of the same compound can be done in different manners.
Answer to Problem 24.39AP
The compounds,
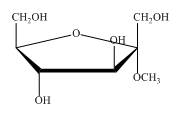
Explanation of Solution
The structure of

Figure 6
So, the compound is same, they are identical. In first name it is taken as methyl derivative of
The compound shown in Figure 6 can be named as
Want to see more full solutions like this?
Chapter 24 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?arrow_forwardThe first step in the metabolism of glycerol, formed by digestion of fats, is phosphorylation of the pro-R—CH2OH group by reaction with adenosine triphosphate (ATP) to give the corresponding glycerol phosphate plus adenosine diphosphate (ADP). Show the stereochemistry of the product.arrow_forwardTrehalose, C12H22O11, is a nonreducing sugar that is only 45% as sweet as sugar. When hydrolyzed by aqueous acid or the enzyme maltase, it forms only d-glucose. When it is treated with excess methyl iodide in the presence of Ag2O and then hydrolyzed with water under acidic conditions, only 2,3,4,6-tetra-O-methyl-d-glucose is formed. Draw the structure of trehalosearrow_forward
- Calculate the degree of unsaturation in each of the following formulas (a) Cholesterol, C27H46o (b) DDT, C14HgC15 (c) Prostaglandin E1, C2oH3405 (d) Caffeine, C8H10N402 (e) Cortisone, C21H28O5 (f) Atropine, C17H23NO3arrow_forwardAll the glucose units in dextran have six-membered rings. When a sample of dextran is treated with methyl iodide and Ag2O and the product ishydrolyzed under acidic conditions, the final products are 2,3,4,6-tetra-O-methyl-d-glucose, 2,4,6-tri-O-methyl-d-glucose, 2,3,4-tri-O-methyl-d-glucose, and 2,4-di-O-methyl-d-glucose. Draw a short segment of dextran.arrow_forwardThe structure of 4 isomers of an aldotetrose carbohydrate are given. 1) select every structure that is a diastereomer of structure D A, B, or C? 2) select every structure that is a enantiomer of structure C D, B, or A? 3) select every structure that is a stereoisomer of structure D A, B, or Carrow_forward
- Trehalose is a nonreducing disaccharide (C12H22O11) isolated from the poisonous mushroom Amanita muscaria. Treatment with an a@glucosidase converts trehalose to two molecules of glucose, but no reaction occurs when trehalose is treated with a b@glucosidase.When trehalose is methylated by dimethyl sulfate in mild base and then hydrolyzed,the only product is 2,3,4,6-tetra-O-methylglucose. Propose a complete structure andsystematic name for trehalose.arrow_forwardGive the full biochemical name for an L-ketohexose with the following pattern of chiral centers: carbon #3 = L, carbon #4 = D.arrow_forwardHow many chiral centers are in B-d-glucopyranose and a-D-galactopyranose? How many stereoisomers of these two aldohexoses can theoretically be drawn?arrow_forward
- Draw the structure of alpha-d-glucopyranose in straight chain cyclic, Haworth and cyclohexane-chair format. Draw the structures of two aldohexoses which are diastereomers but not epimersarrow_forwarddoes structure E represent fructofuranose? explainarrow_forwardDraw Fischer projections for the product(s) formed by reaction of d-ribose with the following. In addition, state whether each product is optically active or inactive Q. C6H5NH2arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


