
Concept explainers
(a)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
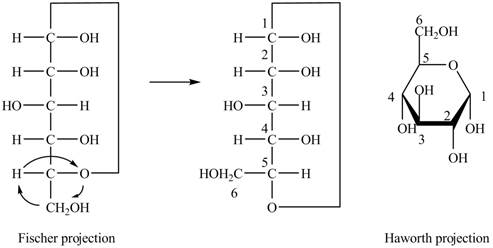
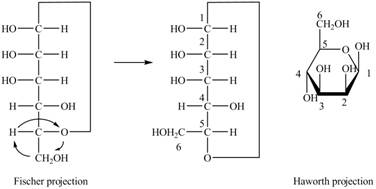
The Fischer projection of
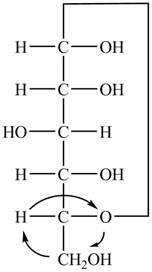
Figure 1
The Fischer projection of
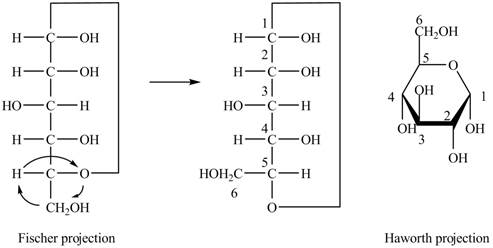
Figure 2
The chair conformation of
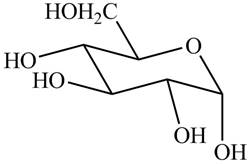
Figure 3
The Fischer projection, Haworth projection, and a line-and wedge structure for
(b)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
The Fischer projection of
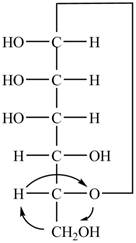
Figure 4
The Fischer projection of

Figure 5
The chair conformation of
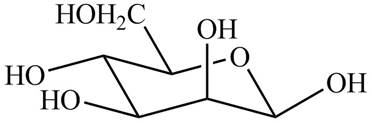
Figure 6
The Fischer projection, Haworth projection, and a line-and wedge structure for
(c)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
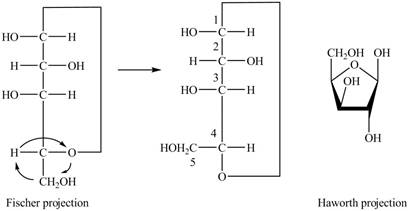
The Fischer projection of
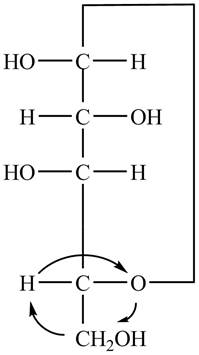
Figure 7
The Fischer projection of
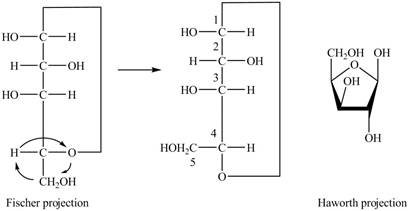
Figure 8
The chair conformation of
The Fischer projection, Haworth projection, and a line-and wedge structure for
(d)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
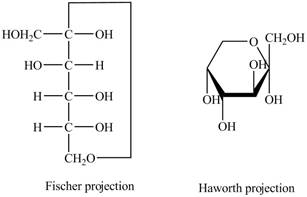
The Fischer projection of
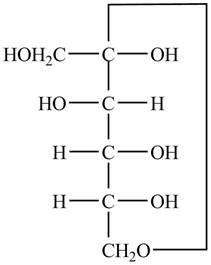
Figure 9
The Fischer projection of

Figure 10
The chair conformation of
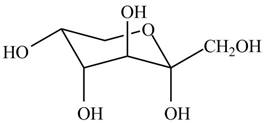
Figure 11
The Fischer projection, Haworth projection, and a line-and wedge structure for
(e)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
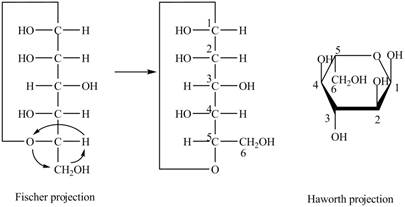
Explanation of Solution
The Fischer projection of
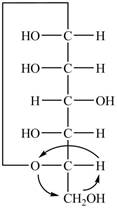
Figure 12
The Fischer projection of

Figure 13
The chair conformation of
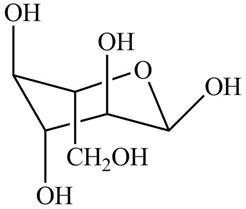
Figure 14
The Fischer projection, Haworth projection, and a line-and wedge structure for
(f)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for a mixture of the
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for a mixture of the
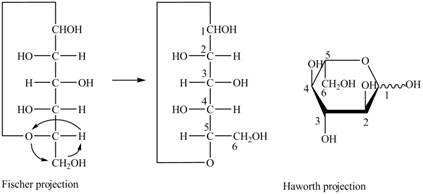
Explanation of Solution
The Fischer projection for a mixture of the
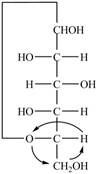
Figure 15
The Fischer projection for a mixture of the

Figure 16
The chair conformation for a mixture of the
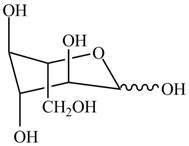
Figure 17
The Fischer projection, Haworth projection, and a line-and wedge structure for
Want to see more full solutions like this?
Chapter 24 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- A What of the following is true about the cyclic structure of scheme A? (a) Pyranose ring ; b) Furanose ring ; c) Alpha anomeric configuration ; d) a and c ; e) b and carrow_forwardHow many chiral centers are in B-d-glucopyranose and a-D-galactopyranose? How many stereoisomers of these two aldohexoses can theoretically be drawn?arrow_forwardA D-aldopentose A is reduced to an optically active alditol. Upon Kiliani–Fischer synthesis, A is converted to two Daldohexoses, B and C. B is oxidized to an optically inactive aldaric acid. C is oxidized to an optically active aldaric acid. What are the structures of A–C?arrow_forward
- The following compound has two asymmetric centers and four stereoisomers. Two of these are d-erythrose and d-threose, which are naturally occurring sugars. The configuration of d-erythrose is (2R,3R), and the configuration of d-threose is (2S,3R). a. Which structure represents d-erythrose? b. Which represents d-threose?arrow_forwarda. Draw a three-dimensional structure for the following steroid. b. What is the structure of the single stereoisomer formed by reduction of this ketone with H2, Pd-C? Explain why only one stereoisomer is formed.arrow_forwarda. What is the classification of the Arabinose in terms of combined no. of carbons and highest functional group present? b. Provide the Cahn-Ingold-Prelog (R.S) Configuration of all the Chiral C present in the structure given above. c. State a Function of arabinose.arrow_forward
- There are three (3) vials labeled A, B, and C known to contain the following monosaccharides. All three samples reduce Tollens and Fehling. By oxidation with dilute HNO3 an optically active aldaric acid is obtained for sample A and the remaining two give products without optical activity. When the three samples were subjected to an alkaline medium, it was observed that, after a certain time, samples A and C reached the same value of the specific rotation [α]. Select the RIGHT alternative: (a) Sample A is Galactose. (b) Sample B is Alosa. (c) Samples A and C are not related to each other by an epimerization process. (d) Sample C is Talose. (e) Samples B and C are epimers.arrow_forwardThe anticoagulant heparin is a polysaccharide that contains alternating residues of -D- glucuronic acid-6- sulfate and N-sulfo-D-glucosamine-6sulfate connected by (1 B 4)- glycosidic linkages. Draw a part of heparin that shows one each of the two residues.arrow_forwardHow many stereoisomers are possible for an aldopentose?arrow_forward
- An unknown β-d-aldohexose has only one axial substituent. A Wohl degradation forms a compound which, when treated with sodium borohydride, forms an optically active alditol. This information allows you to arrive at two possible structures for the β-d-aldohexose. What experiment can you carry out to distinguish between the two possibilities?arrow_forwarda. In an aqueous solution, d-glucose exists in equilibrium with two six-membered ring compounds. Draw the structures of these compounds.b. Which of the six-membered ring compounds will be the major product?arrow_forwardA hexose was obtained after (+)-glyceraldehyde underwent three successive Kiliani–Fischer syntheses. Identify the hexose from the following experimental information: oxidation with nitric acid forms an optically active aldaric acid; a Wohl degradation followed by oxidation with nitric acid forms an optically inactive aldaric acid; and a second Wohl degradation forms erythrose.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





