
(a)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Amino group reacts faster with the
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
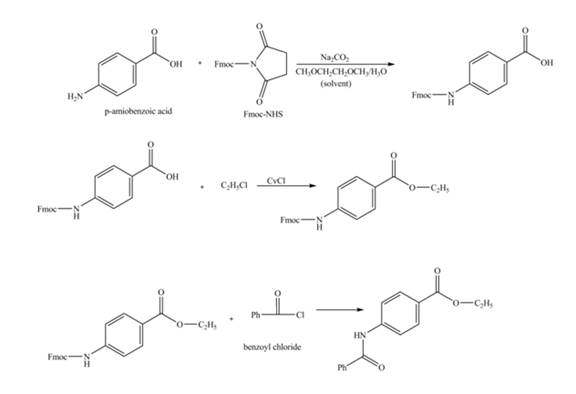
Explanation of Solution
The given reaction is shown below.
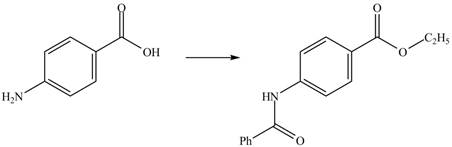
Figure 1
The amine group of the
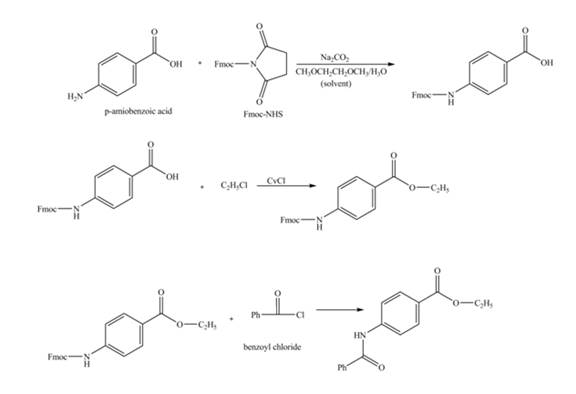
Figure 2
The complete synthesis of the given compound is shown in Figure 2.
(b)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Sodium borohydride is a strong reducing agent. The hydride ion of sodium borohydride acts as the nucleophile and gets attached to the carbonyl carbon.
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
Explanation of Solution
The given reaction is shown below.
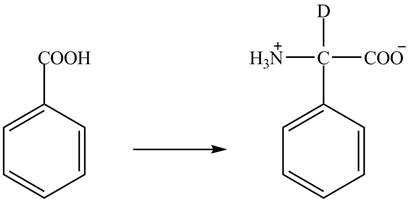
Figure 3
The benzoic acid reacts with
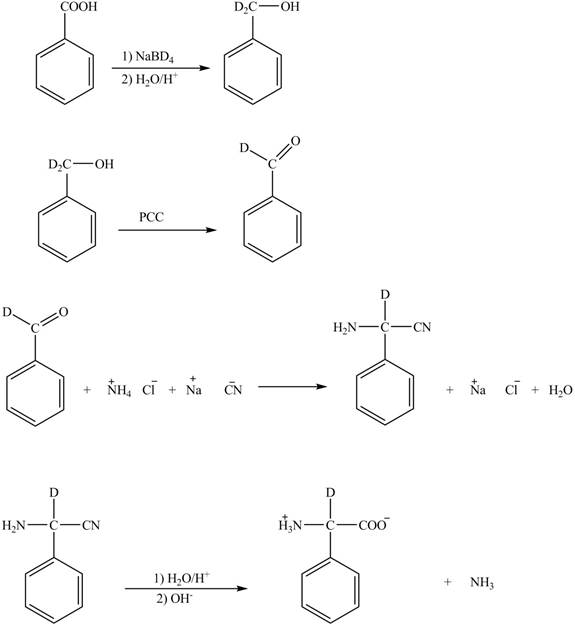
Figure 4
The complete synthesis of the given compound is shown in Figure 4.
(c)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Aldehydes reacts with sodium cyanide to form nitrile compound. The nitrile compound reacts with ammonium chloride to form the
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
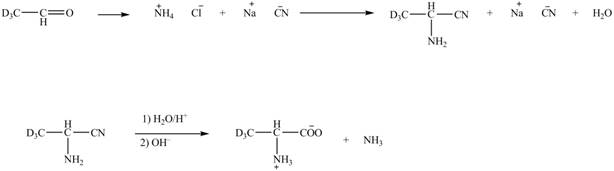
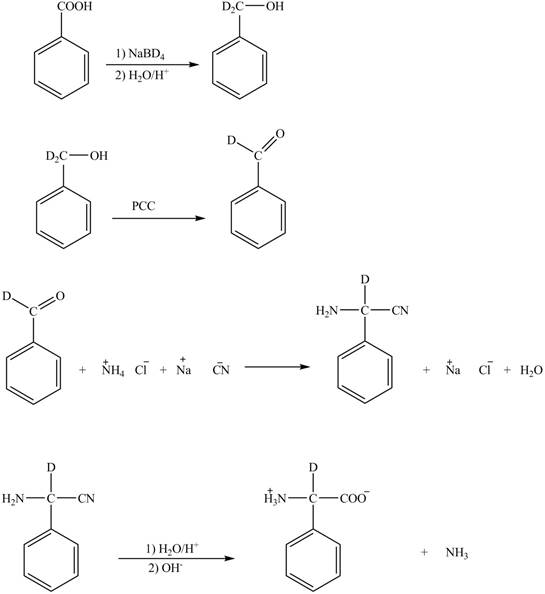
Explanation of Solution
The given reaction is shown below.
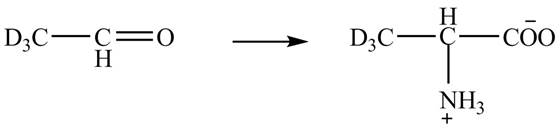
Figure 5
The deuteriated acetaldehyde reacts with ammonium chloride and sodium cyanide to form deuteriated
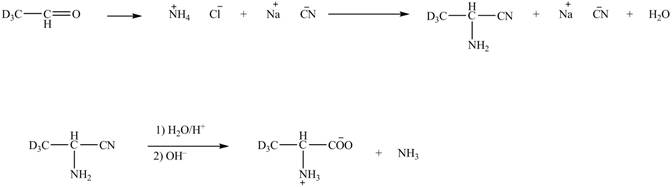
Figure 6
The synthesis of the given compound is shown in Figure 6.
(d)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Amino group reacts faster with the alkyl halides than the carboxylic group. The reaction of carboxylic acid can be performed only by protecting the amine group. Fmoc compound is used as the protecting agent for amine group. Amine and acid chloride reacts to form amide bond.
Answer to Problem 27.71AP
The synthesis of given compound from the given starting material is shown below.
Explanation of Solution
The given reaction is shown below.
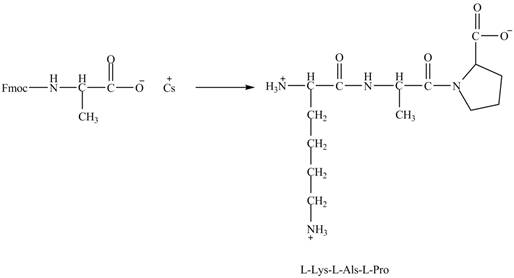
Figure 7
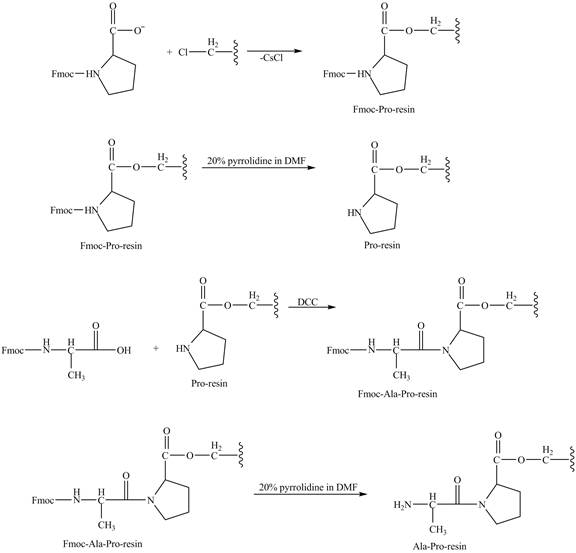
The cesium salt of Fmoc-proline is reacted with the resin linker. The amine is deprotecteed in pyrrolidine. Fmoc-alanine reacts with the prolin resin in presence of
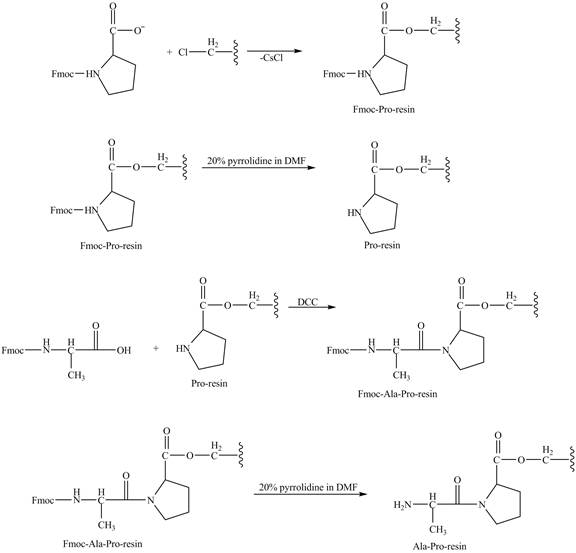
Figure 8
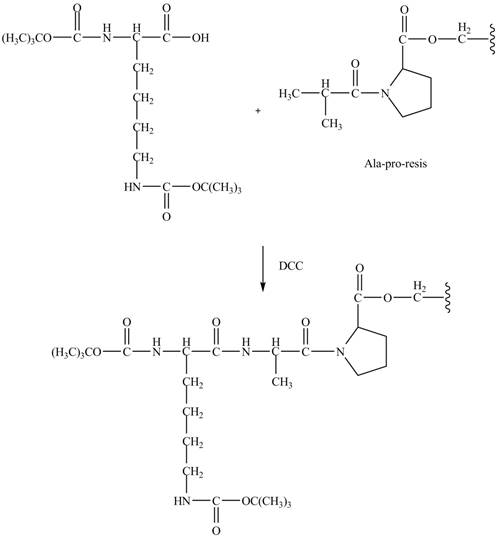
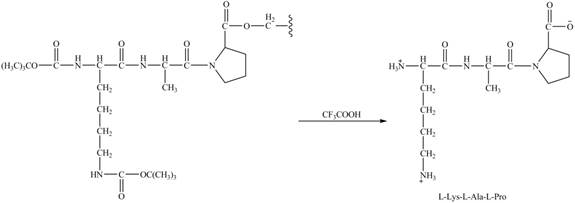
The alanine-proline resin reacts with the Boc protected lysin to form the
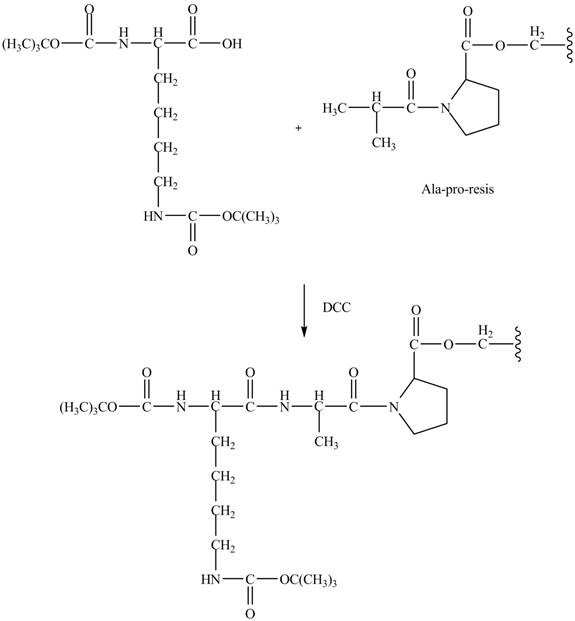
Figure 9
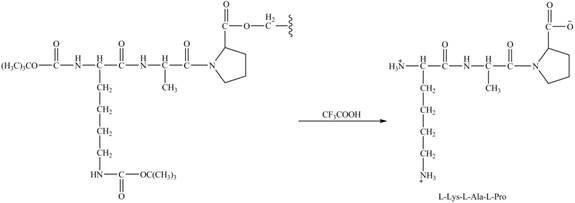 Figure 10
Figure 10
The synthesis of the given compound is shown in Figure 8 and Figure 9 and Figure 10.
(e)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
Aldehydes reacts with sodium cyanide to form nitrile compound. The nitrile compound reacts with ammonium chloride to form the
Answer to Problem 27.71AP
The complete synthesis of given compound is shown below.
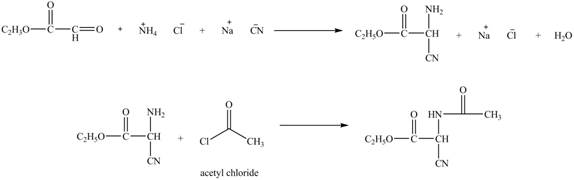
Explanation of Solution
The given reaction is shown below.
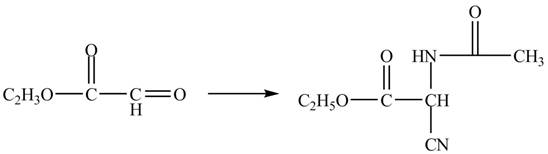
Figure 11
The compound
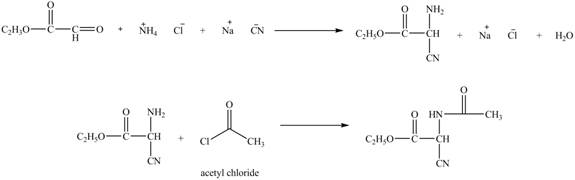
Figure 12
The synthesis of the given compound is shown in Figure 12.
(f)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
The
Answer to Problem 27.71AP
The complete synthesis of given compound is shown below.
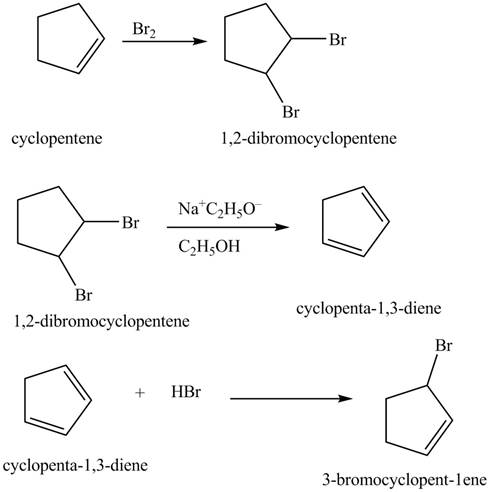
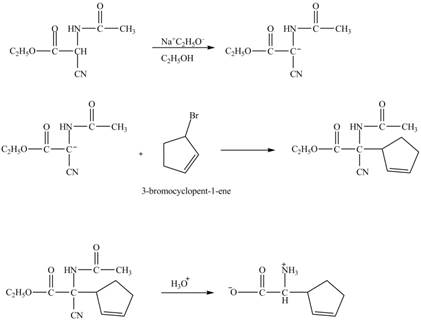
Explanation of Solution
The given reaction is shown below.
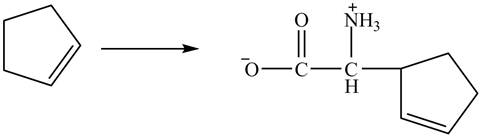
Figure 13
The compound cyclopentene undergo bromination reaction to form
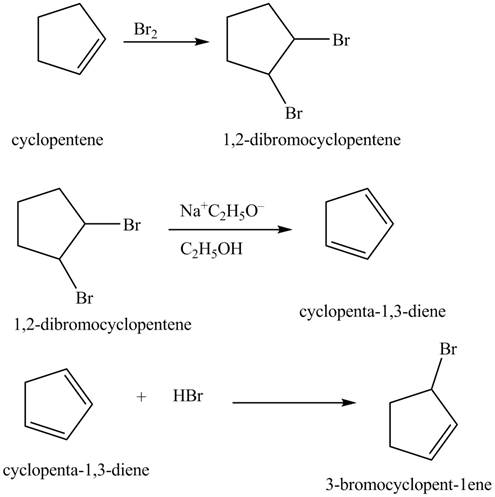
Figure 14
The given nitrile compound upon reaction with sodium ethoxide forms anion. The formed anion reacts with
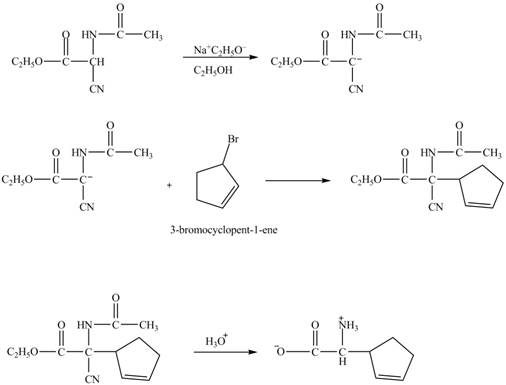
Figure 15
The complete synthesis of given compound is shown in Figure 14 and Figure 15.
(g)
Interpretation:
The synthesis of given compound from the given starting material is to be stated.
Concept Introduction:
The reaction of carboxylic acid and amines to form amide is not possible. Amines are very basic so it abstracts acidic proton from the carboxylic acid. The reaction is performed in the presence of
Answer to Problem 27.71AP
The complete synthesis of given compound is shown below.
Explanation of Solution
The given reaction is shown below.
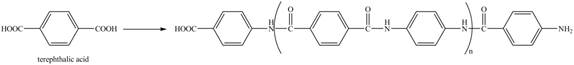
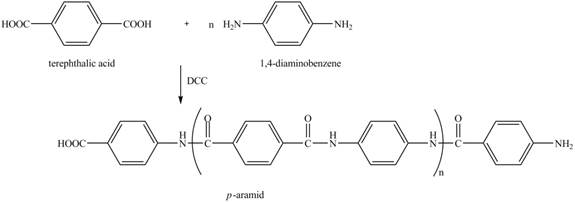
Figure 16
The reaction between terephthalic acid and

Figure 17
The synthesis of given compound is shown in Figure 17.
Want to see more full solutions like this?
Chapter 27 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Show the peptides that would result from cleavage by the indicated reagent: Arg-Ser-Pro-Lys-Lys-Ser-Glu-Gly by trypsinarrow_forwardAn amino acid mixture of phenylalanine, glycine and glutamic acid is to be separated bypaper chromatography. The solvent is less polar than water. Which of these amino acids will have the highest Rf value and which the lowest? Explain. Treatment of a new protein with dansyl chloride reveals two (2) dansyl-labelled derivatives of amino acids, alanine and methionine. What can you deduce about the structure of the protein from these results? Name the amino acids that contribute atoms to both purine and pyrimidine rings.arrow_forwardBradykinin is a linear nonapeptide released by blood plasma globulin in response to a wasp sting. It is a very potent pain-causing agent. Its constituent amino acids are 2R, G, 2F, 3P, S. The sue of 2,4-dinitrofluorobenzene and carboxypeptidase shows that both terminal residues are arginine. Partial hydrolysis of bradykinin gives the following di- and tripeptides: FS + PGF +PP + SPF + FR + RParrow_forward
- Protein: SHAYNERSE Predict the products of the following reactions with the protein given, if there is none, write NO RXN. - Biuret reagent - KOH/Pb(CH2COO)2 - Glyoxilic Acid/Conc. H2SO - Hg/HNO3 - HNO3arrow_forwardBy using only amide/Peptide bond forming rxn and a lKylation rxns. Provide all the reaction steps with the reagent needed and conditions with out showing the mechanism for each step to form the product. Note: Use protecting groups like (Boc, Fmoc, and others) H2NNH2+arrow_forwardWrite the form of lysine most prevalent at pH 1.0 and then show its reaction with the following. Consult Table 27.2 for pKa values of the ionizable groups in lysine. Q.) 1 mol NaOHarrow_forward
- An unknown decapeptide was isolated and characterized. Complete hydrolysis of this peptide gave : F(2), A,G,C,K,N,T, W and V. Treatment with carboxypeptidase releases A. Reaction with Edman’s reagent gave PTH-T and a nonapeptide. The nonapeptide was treated with trypsin and gave 2 peptides: (V-C-G-A) and (N-FF-W-K). Give the sequence of amino acid in the decapeptide.arrow_forwardShow the peptides that would result from cleavage by the indicated reagent: Val-Arg-Gly-Met-Arg-Ala-Ser by carboxypeptidase Aarrow_forwardWhat would the overall charge of this peptide sequence (Asp-Ala-Phe-Lys-His) at pH 12 be? Give a clear handwritten answer with explanation..please give explained answerarrow_forward
- The Arg-Gly-Asp tripeptide (RGD) is a well-known tumour targeting peptidemotif. Explain how you would synthesise H-Arg-Gly-Asp-OH, starting fromthe constituent amino acids? Explain all steps that are necessary and discuss amechanism for one side chain protection and one C terminus protection.arrow_forwardWhat would be the net charge on the peptide Asn-Phe-Lys at pH = 10.54?arrow_forwardWhat are the experimental procedures and the laboratory equipments needed to be used in the removing of carbon dioxide and Nucleophilic addition on the nitrogen of isoxazoline that will have a product of protein that contain native serine residues by the reaction of oxazetidine-containing peptides and α-ketoacidarrow_forward
