
Concept explainers
(a)
Interpretation:
The following pericyclic reaction is to be classified. (More than one classification is possible.)
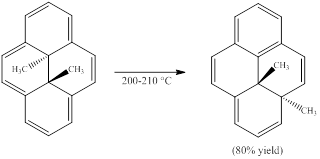
Concept introduction:
Sigmatropic reaction can be described as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. In some cases, Lewis acid catalysts can also be applied. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement.
(b)
Interpretation:
Suppose the migrating methyl group in part (a) were labeled with the hydrogen isotopes deuterium (D) and tritium (T) so that it is a -CHDT group with the S configuration. The configuration of this group in the product is to be stated.
Concept introduction:
Sigmatropic reaction can be described as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. In some cases, Lewis acid catalysts can also be applied. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
- (A) what are the 'Four criteria (rules) for aromaticity'? (b) Explain aromatic, antiaromatic, nonaromatic using 'Criteria for aromaticiy' and draw chemical structures of each example.arrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardAlkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two in-plane bonds per each chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of the reactions. Be sure to draw hydrogens on oxygen, where applicable. Select Draw Rings More Erase C 1. B2H6, diglyme (a) 2. H2O2, HO¯, H2Oarrow_forward
- Alkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two in-plane bonds per each chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of the reactions. Be sure to draw hydrogens on oxygen, where applicable. Select Draw Rings More Erase H 1. B,H§, diglyme (a) 2. H2O2, HO", H2Oarrow_forwardAlkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two in-plane bonds per each chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of the reactions. Be sure to draw hydrogens on oxygen, where applicable. 1. B2H6, diglyme (a) 2. H202, HO¯, H20 OH OH H. Incorrect MacBook Proarrow_forwardAlkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two in-plane bonds per each chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of the reactions. Be sure to draw hydrogens on oxygen, where applicable. H 1. B2H6. diglyme (a) H 2. H2O2, HO-, H20 он OH Incorrectarrow_forward
- Alkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two in-plane bonds per each chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of the reactions. Be sure to draw hydrogens on oxygen, where applicable. Select Draw Rings More Erase H 1. B₂H6, (a) diglyme 2. H2O2, HOT, H₂O ✓ C O Q2 Qarrow_forwardA reasonable synthesis for each of the following compoundsarrow_forward(i) Explain why a high reaction temperature favours elimination reactions, instead of substitution reactions. (ii) Explain why polar aprotic solvents favour Sn2 reaction but not favour SN1 reaction.arrow_forward
- Which of (a)-(d) is not aromatic? (B) (A) (C) (D)arrow_forwardAlkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two O Hint in-plane bonds per each chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of the reactions. The first step of the reaction, hydroboration, involves addition of BH, to the double bond, with -BH, attached to one carbon and hydrogen Be sure to draw hydrogens on oxygen, where applicable. attached to the other. Consider both Rings the regioselectivity and the Select Draw More Erase stereochemistry of this addition. H Diborane, B,H,, is a source of borane, BH,, and can be used 1. B2H6, interchangeably. Diglyme is a solvent. diglyme 2. H2О2, НО", The product or products of the reaction are characterized as being O S. O racemic. O achiral. O R,S (and/or S,R). O R. O S,S. O R.R. O diastereomers.arrow_forwardPlease don't provide handwritten solution .....arrow_forward
