
Concept explainers
(a)
Interpretation:
Explain why the following equilibrium lies far to the right.
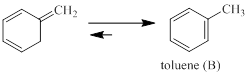
Concept introduction:
In chemistry, resonance is a systematic way to describe bonding nature of certain molecules of ions (cation or anion) by combining several structures which are contributing to the core structure. The various structures derived for a molecule is referred as resonance structures or canonical structures.
(b)
Interpretation:
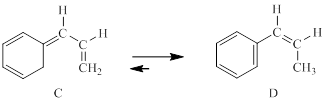
Chemists had always assumed that this reaction would be so fast that compound A could never be isolated. However, this compound was prepared in 1962 and shown to be stable in the gas phase at 70°C, despite the favorable equilibrium constant for its transformation to B.Show why the conversion of A into B above would not be expected to occur as a concerted reaction.
Concept introduction:
Generally, Sigmatropic reaction is referred as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement.
(c)
Interpretation:
The concerted mechanism for the following reaction is to be explained.
Concept introduction:
Generally, Sigmatropic reaction is referred as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
- Rewrite each of the following stereospecific transforms as a synthetic step in the forward direction, including reagents and any special reaction conditions. (a) (b) OH "CH3 CH3 H (Racemic) (c) OH (d) CN Br (Racemic) Brarrow_forward(c) Compound 2F shown below is a tetra-substituted cyclooctatetraene. After heating 2F at 160 degree for 6 hours, researchers observed the formation of an isomer of 2F. Draw the isomer structure and provide a plausible mechanism to explain the formation of the isomers. 2Farrow_forward(a) Name the reactant (including E/Z configuration) and draw the structure of the major product for the following reaction. + HBr (b) Explain your answer for the product in part (a), and include the structure of the carbocation intermediate in your explanation. (c) Draw the mechanism for the reaction in part (a), showing all intermediates and electron movement with arrows.arrow_forward
- Consider the addition of HBr shown here. HBr (a) There are three carbocation intermediates possible from the protonation of this triene. Draw all three of them and identify the most stable one. (b) Draw all halogenated products formed by attack of Br on the most stable carbocation. (c) Which of those products would you expect to be formed in the greatest amount at low temperatures? (d) Which would you expect to be formed in the greatest amount at high temperatures?arrow_forwardIt has been observed that the reaction of B to produce Product D proceeds 10,000X faster than the reaction of A to produce Product C. Provide a brief explanation for the difference in observed rates.arrow_forwardWhen ethyl bromide is added to potassium tert-butoxide, the product is ethyl tert-butyl ether.(a) What happens to the reaction rate if the concentration of ethyl bromide is doubled?arrow_forward
- PRACTICE PROBLEM 8.20 Specify the alkene and reagents needed to synthesize each of the following diols. OH HO- (a) (b) (c) HO. н он HO (racemic) (racemic)arrow_forwardPredict the product(s) and show the complete electron-pushing mecha- nism for each of the following dissolving metal reductions. (a) H3CC=CCH₂CH3 (b) (c) CECH 1844 Li NH3 ND3 C=C—CH3 NH3arrow_forwardName each alkene and specify its configuration by the E,Z system. (Be sure to indicate double bond stereochemistry using (E)/(Z) notation. It is not necessary to use italics in writing compound names.) (a) (b) Brarrow_forward
- (a) Decide whether the reaction below will proceed via an E1 or E2 and write a step-by-step mechanism. (b) Draw structural formula from the major organic product only.arrow_forwardDiscuss the hybridization, aromaticity, and stability of the following organic intermediate. Also, arrange them in the increasing order of stability. CHarrow_forwardDraw a structural formula for the alcohol formed by treating each alkene with borane in tetrahydrofuran (THF) followed by hydrogen peroxide in aqueous sodium hydroxide, and specify stereochemistry where appropriate. (a) (d) (b) (e) (c)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





