
Concept explainers
(a)
Interpretation:
The type of pericyclic reaction required to form benzene from Dewar benzene is to be stated.
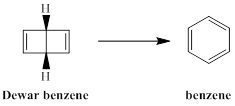
Concept introduction:
The isomer of benzene having bicyclic ring with the molecular formula of C6H6 is termed as Dewar benzene or bicyclo [2, 2, 0] hexa-2, 5-diene. This structure is included in the list of possible structure of benzene and named after James Dewar. However, this bicyclic ring structure is not accepted as the true structure of benzene and Dewar supported the Kekule structure of benzene.
(b)
Interpretation:
Dewar benzene, although a very unstable molecule, is not readily transformed to benzene is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time. Notably, the pericyclic reactions are classified as electrocyclic, cycloaddition and sigmatropic reactions based on the activation of reaction, the number of electrons involved and the stereochemistry outcome of the reactions.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
- Disiamylborane adds only once to alkynes by virtue of its two bulky secondary isoamylgroups. Disiamylborane is prepared by the reaction of BH3 # THF with an alkene.(a) Draw the structural formulas of the reagents and the products in the preparation ofdisiamylborane.(b) Explain why the reaction in part (a) goes only as far as the dialkylborane. Why isSia3B not formed?arrow_forwardDraw the structure of the following compounds all showing C and H atoms.(a) 2-methyl -3-iso propyl heptanes(b) Dicyclopropyl methane.arrow_forwardBiphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?(c) The heat of hydrogenation for biphenyl is about 418 kJ>mol (100 kcal>mol). Calculate theresonance energy of biphenyl.(d) Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain thedifference in the resonance energies of naphthalene and biphenyl.arrow_forward
- Explain why (i) the dipole moment in chlorobenzene is lower than that of cyclohexyl chloride. (ii) haloalkanes are only slightly soluble in water but dissolve easily in organic solvents.arrow_forward(a) When cyclopropane is treated with HI, 1-iodopropaneis formed. A similar type of reaction does not occur withcyclopentane or cyclohexane. Suggest an explanation forcyclopropane’s reactivity. (b) Suggest a method of preparingethylbenzene, starting with benzene and ethylene asthe only organic reagents.arrow_forward(a) Draw a specific organic reaction for the halohydrin formation addition reaction of alkenes. (b) Also write the reagents involved in the reaction (c) and name and identify its major and minor product by writing its IUPAC name.arrow_forward
- Which of the following is true for the reactions of alkyl halides? (a) The characteristic reactions of alkyl halides are oxidation and reduction.(b) The characteristic reactions of alkyl halides are elimination and substitutionc. The characteristic reactions of alkyl halides are addition and substitutiond. Characteristic reactions of alkyl halides are addition and elimination.arrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 2-cyclopropoxypentane;(b) 1,2-dimethoxy-4-propylcyclohexane; (c) 4-(1,1-dimethylethyl)-1,2-dipropoxycyclooctanearrow_forwardAcid-catalyzed dehydration of 2,2-dimethyl-1-hexanol gave a number of isomeric alkenes including 2-methyl-2-heptene as shown in the following equation.(a) Write a stepwise mechanism for the formation of 2-methyl-2-heptene, using curved arrows to show the flow of electrons. (b) What other alkenes do you think are formed in this reaction?arrow_forward
- trans-3-hexene and cis-3-hexene differ in one of the following ways. Which one? (A) Products of hydrogenation (B) Products of ozonlysis (C) Products of bromine (Br2) addition (D) Products of hydroboration-oxidationarrow_forwardH H Write the structures of the alkenes that would yield the following carbonyl compounds PRACTICE PROBLEM 8.22 when treated with ozone and then with dimethyl sulfide. (a) and (c) EO and `H oalio (2 mol is produced from 1 mol of alkene) (b) H.arrow_forward1) The carbon-oxygen double bond present in aldehydes and ketones is very polar. What does this mean and how does it arise? 2) The carbon-oxygen double bond is readily attacked by nucleophiles like cyanide ions or ammonia. (i) What do you understand by the term nucleophile? (ii) Which part of the carbon-oxygen double bond is attractive to nucleophiles? 3) Why is there a difference between aldehydes and ketones in their response to oxidizing agents such as potassium dichromate(VI) solution acidified with dilute sulfuric acid?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





