
Concept explainers
Draw the products formed when D-altrose is treated with each reagent.
a.
b.
c.
d.
e. [1]
f. [1]
g.
h.
(a)
Interpretation: The products formed by the treatment of D-altrose with
Concept introduction: The
Answer to Problem 28.50P
The products formed by the treatment of D-altrose with
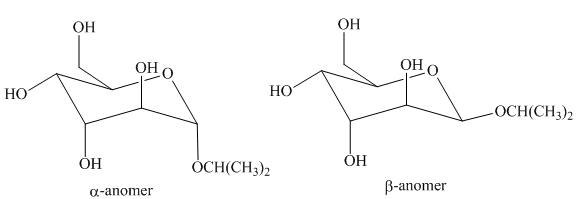
Figure 1
Explanation of Solution
The
The products formed by the treatment of D- altrose with
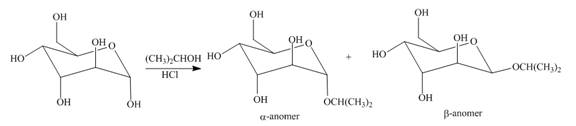
Figure 2
The products formed by the treatment of D-altrose with
(b)
Interpretation: The products formed by the treatment of D-altrose with
Concept introduction: The substitution reaction involves the replacement of one functional group by other functional group. In nucleophilic substitution an electron rich species attack the species that is deficient in electrons. The electrophile and the leaving group together form a substrate. The nucleophile attacks over the substrate and there occurs the removal of leaving group from the substrate.
Answer to Problem 28.50P
The product formed by the treatment of D-altrose with
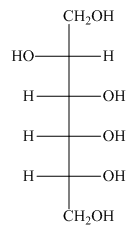
Figure 3
Explanation of Solution
The product formed by the treatment of D-altrose with
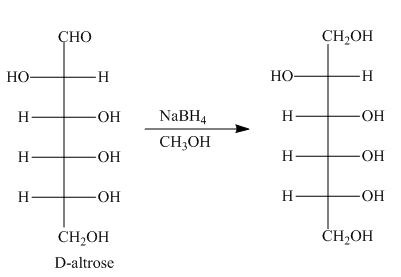
Figure 4
In the given reaction, sodium borohydride is used as a reducing agent. Sodium borohydride is used to for the reduction of carbonyl compounds to alcohols.
The product formed by the treatment of D-altrose with
(c)
Interpretation: The products formed by the treatment of D-altrose with
Concept introduction: The aldehyde group of aldoses oxidizes to carboxyl group on treatment with
Answer to Problem 28.50P
The product formed by the treatment of D-altrose with
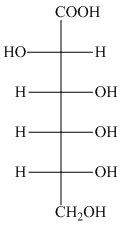
Figure 5
Explanation of Solution
The oxidation of aldehyde group of aldoses leads to the formation of aldonic acid, in which the terminal carbon atoms are substituted by carboxyl group and alcoholic group. The product formed by the treatment of D-altrose with
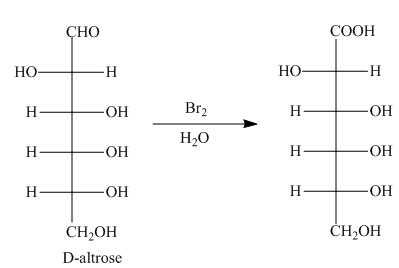
Figure 6
The product formed by the treatment of D-altrose with
(d)
Interpretation: The products formed by the treatment of D-altrose with
Concept introduction: The aldehyde group and primary alcohol of aldoses oxidizes to carboxyl groups on treatment with warm
Answer to Problem 28.50P
The product formed by the treatment of D-altrose with
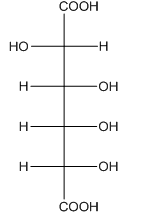
Figure 7
Explanation of Solution
The oxidation of aldehyde group and primary alcohol of aldoses leads to the formation of aldaric acid, in which the terminal carbon atoms are substituted by carboxyl groups. The product formed by the treatment of D-altrose with
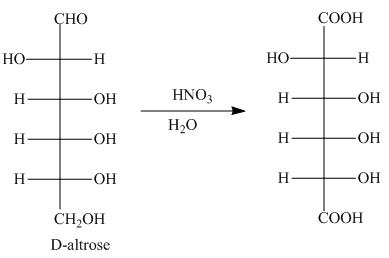
Figure 8
The product formed by the treatment of D-altrose with
(e)
Interpretation: The products formed by the treatment of D-altrose with [1]
Concept introduction:
Answer to Problem 28.50P
The product formed by the treatment of D-altrose with [1]
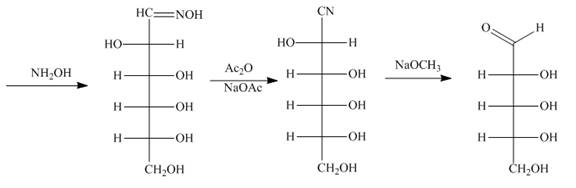
Figure 9
Explanation of Solution
The reaction of D-altrose with hydroxylamine results in the formation of oxime. The second step is the dehydration of oxime to nitriles. The reaction of nitrile with
The product formed by the treatment of D-altrose with [1]
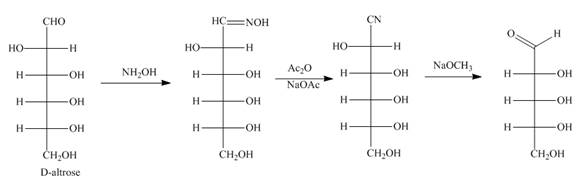
Figure 10
The product formed by the treatment of D-altrose with [1]
(f)
Interpretation: The products formed by the treatment of D-altrose with [1]
Concept introduction:
Answer to Problem 28.50P
The products formed by the treatment of D-altrose with [1]
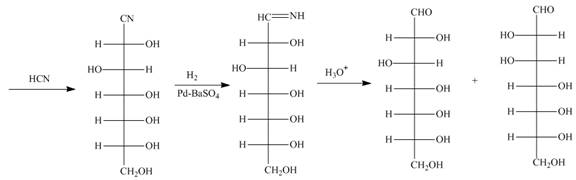
Figure 11
Explanation of Solution
The products formed by the treatment of D-altrose with [1]
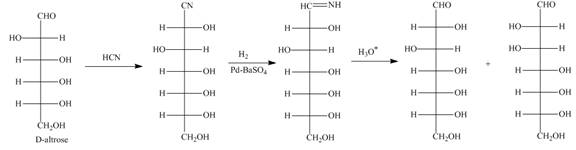
Figure 12
The products formed by the treatment of D-altrose with [1]
(g)
Interpretation: The products formed by the treatment of D-altrose with
Concept introduction: The
Answer to Problem 28.50P
The products formed by the treatment of D-altrose with
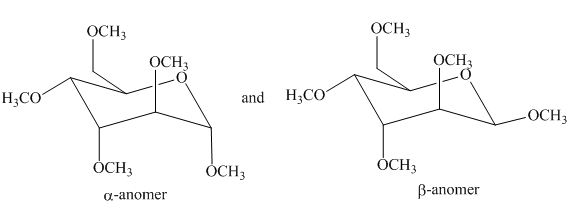
Figure 13
Explanation of Solution
The
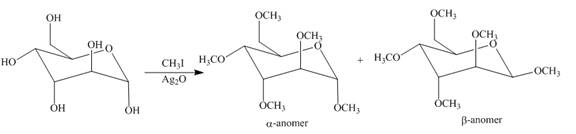
Figure 14
The products formed by the treatment of D-altrose with
(h)
Interpretation: The products formed by the treatment of D-altrose with
Concept introduction: The
Answer to Problem 28.50P
The products formed by the treatment of D-altrose with
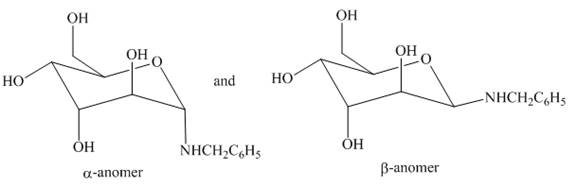
Figure 15
Explanation of Solution
The
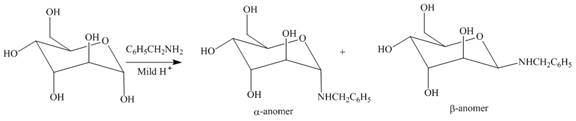
Figure 16
The products formed by the treatment of D-altrose with
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardDraw the product formed when C6H5N2 +Cl− reacts with each compound.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





