
Concept explainers
Interpretation:
The two product in the given reaction are formed after [1, 7] sigmatropic rearrangement, one due to hydrogen migration and the other to deuterium migration. It should be represented the configuration of product by replacing the A and B with appropriate atoms (H or D)
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
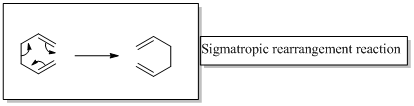
Sigma tropic rearrangement reactions are designated with digits. For example a [1, 3] sigma tropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for sigma tropic rearrangement reactions are listed below
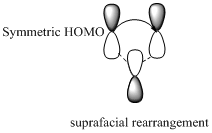
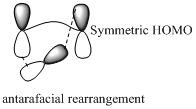
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction. Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Carbon migrating with one lobe of its p orbital interacting

Carbon migrating with both lobe of its p orbital interacting

Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
ORGANIC CHEMISTRY W/ST.GDE W/ACCESS
- Consider the reactions shown below. For each reaction state if it would favor S N 1 or S N 2pathway and give the major product of the reaction.arrow_forwarda) What product is formed from the [1,7] sigmatropic rearrangement of a deuterium in the following triene? (b) Does this reaction proceed in a suprafacial or antarafacial manner under thermal conditions? (c) Does this reaction proceed in a suprafacial or antarafacial manner under photochemical conditions?arrow_forwardA student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br. Draw the products obtained from each free-radical intermediate.arrow_forward
- In a strongly acidic solution, cyclohexa-1,4-diene tautomerizes to cyclohexa-1,3-diene.Propose a mechanism for this rearrangement, and explain why it is energetically favorable.Parrow_forwardWhen Br2 is added to buta-1,3-diene at -15 °C, the product mixture contains 60% ofproduct A and 40% of product B. When the same reaction takes place at 60 °C, theproduct ratio is 10% A and 90% B.If you had a solution of pure A, and its temperature were raised to 60 °C, what wouldyou expect to happen? Propose a mechanism to support your prediction.arrow_forwardWhich of the following reagent best accomplish this transformation below? a. BH3, THF, H2O2 b. NaNH2, NH3 c. H2SO4, H2O, HgSO4 d. H2, Lindlararrow_forward
- most of the additions of bromine to double bonds gave entirely antistereochemistry. Explain why the addition to phenanthrene gives a mixture of synand anti stereochemistry. When the product from (c) is heated, HBr is evolved and 9-bromophenanthrene results.Propose a mechanism for this dehydrohalogenation.arrow_forward1. (1-bromo-1, 3-dimethylcyclopentane) an optically active pure sample reacted with water. Write the complete mechanism which includes the electron-pushing arrows. Show the expected stereochemistry for each of the case and each mechanism should give two distinct products and then describe the relationship of the products to one another.arrow_forwardWhat type of sigmatropic rearrangement is illustrated in each reaction?arrow_forward
- Provide a detailed step-wise mechanism for the following reaction. Be sure to show all steps, intermediates, formal changes, and show the movement of electrons with curved arrows. You do not need to show transition states.arrow_forwardFirst Writedown which reaction it is? SN1, SN2, E1? Write a stepwise mechanism for the following reactions showing ALL intermediates. Use curved arrows to symbolize the flow of electrons to show how each of the intermediates and product are formed. Show all necessary lone pairs and formal charges.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

