
(a)
Interpretation:
A mechanism should be proposed for the given reaction.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”
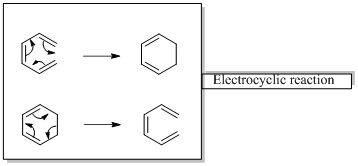
Diels-alder reaction is a cycloaddition reaction which is occurs between a conjugated diene and substituted
Mechanism gives the step wise processes occurs in a particular reaction.
(b)
Interpretation:
The product should be determined for the given reaction if trans-2-butene were used instead of ethene.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”

Diels-alder reaction is a cycloaddition reaction which is occurs between a conjugated diene and substituted alkene to form cyclohexene ring system. This concerted reaction can be accelerated by heating or using some catalysts.
Mechanism gives the step wise processes occurs in a particular reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
ORGANIC CHEMISTRY W/ST.GDE W/ACCESS
- Rank the following dienophiles in their rate of reaction with the same diene in a Diels-Alder reaction. For example, 1 = fastest or most reactive dienophile, 4 = slowest or least reactive dienophile.arrow_forwardDetermine reagents C and D from the reaction scheme below.arrow_forwardshow the mechanism and draw the diels alder product of 1,3 butadiene reacted with cis butenedioic acidarrow_forward
- Could someone help me with ranking these with how reactive they would be with an SN2 reaction? Thanks :)arrow_forwardPlease answer both parts to the questions completely. I will rate the answer afterwards. For a diels-alder reaction between anthracene and maleic anhydride, are the exo and endo forms of product 9,10-dihydroanthracene-9,10-ɑ,β-succinic acid anhydride different stereoisomers or are they the same molecule? Explain your answer by drawing the molecule. Would it be favorable to get a 1,4-adduct of anthracene and maleic anhydride? Why or why not? If the 1,4-adduct of anthracene and maleic anhydride had formed, would it have different exo and endo isomers? Yes or no and why?arrow_forwardRank the following in order from slowest to fastest rate of reaction in a Diels–Alder reaction with buta-1,3-dienearrow_forward
- Rank the attached dienophiles in order of increasing reactivity.arrow_forwardProvide an explanation for why the stereoisomers shown below would not be produced in the Diels-Alder reaction..arrow_forwardAny help with these would be great! Thanks in advance:) What is the major product of the reactions?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
