
(a)
Interpretation: The kind of sigmatropic rearrangement that occurs in each of the given reactions has to be given.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
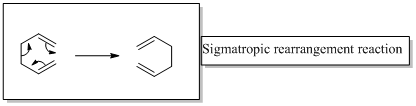
Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
(b)
Interpretation: Using arrows the electron rearrangement takes place in the given reactions has to be shown.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”

Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
- List the following carbocation intermediates in order from least stable to most stable. Explain why they were ranked this way.arrow_forwardWhy does Hammett Equation only apply to meta and para substituted rings and not others? Explainarrow_forwardComplete the following for the below reaction. i. draw the complete Molecular Orbital diagram with electron filling, nodes and phasing. ii. label each level with the number of bonding and antibonding interactions iii. label each level as bonding, nonbonding or antibonding, and HOMO & LUMO iv. Using the correct level, show how the cyclization occurs to give cyclohexadiene and indicate whether the product is cis or trans. heatarrow_forward
- In case of dihalide like problem 8.54 c. 1. my think is like this. at the first E2 reaction, the hydrogen on cyclohexyl will be participate because its carbon is more substituted. then second E2 reaction, there are no more hydrogen on left carbon. so hydrogen on right carbon will be partcipate. as a result, the product is double bond not triple bond. Why is this wrong and only form triple bond? 2. Is it impossible to make Sn2 reaction that NH2- is working as nucleophile?arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. Ⓒ. CCl₂ • You do not have to consider stereochemistry. . Include all valence lone pairs in your answer. . In cases where there is more than one answer, just draw one. Bo H₂SO4 First stage in synthesis of the insecticide DDT, which is now banned in the US #₂ SAIF CI OH CC13arrow_forwardDraw the alkene that would react with the reagent given to account for the product formed. OH H₂SO4 CH3CH2CCH2CH3 ? + H₂O CH2CH3 . You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forward
- 6., Draw the diene and dienophile which would be used to prepare the following bicyclic compound. Label the diene and dienophile. A NO₂ H H NO₂arrow_forwardDraw the products of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry. Ignore inorganic byproducts. 1. Os04 2. NaHSO3, H3O Select to Draw Select to Drawarrow_forwardPls draw clearly. thank youarrow_forward
- 4. Explain why one of the carbocations below is much more stable than the other. Illustrate with an appropriate orbital overlap picture. stable unstablearrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction.arrow_forwardA Moving to another question will save this response. Question 12 Identify the electrophile in a Friedel-Crafts alkylation (remember: the one that will react with the benzene ring) O aluminum tetrachloride ion O radical O carbocation O aluminum chloride O carbanion A Moving to another question will save this response.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
