
Concept explainers
What is the predominant form of each of the following amino acids at
(a)
Interpretation: The predominant form of threonine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.36P
The predominant form of threonine at
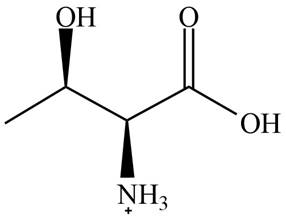
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of threonine is
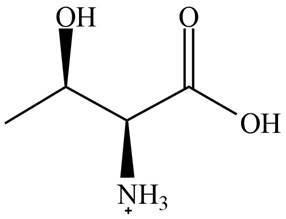
Figure 1
The overall charge on threonine at
The predominant form of threonine at
(b)
Interpretation: The predominant form of methionine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.36P
The predominant form of methionine at
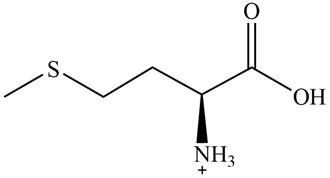
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of methionine is
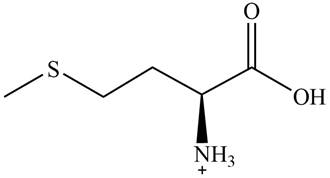
Figure 2
The overall charge on methionine at
The predominant form of methionine at
(c)
Interpretation: The predominant form of aspartic acid at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.36P
The predominant form of aspartic acid at
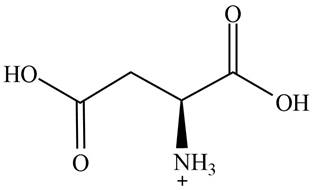
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of aspartic acid is
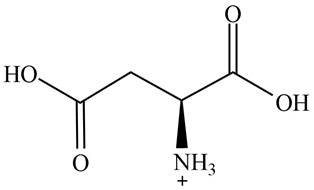
Figure 3
The overall charge on aspartic acid at
The predominant form of aspartic acid at
(d)
Interpretation: The predominant form of arginine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.36P
The predominant form of arginine at
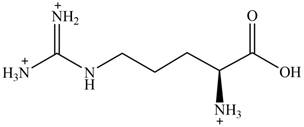
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of arginine is
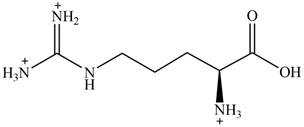
Figure 4
The overall charge on arginine at
The predominant form of arginine at
Want to see more full solutions like this?
Chapter 29 Solutions
ORGANIC CHEMISTRY
- For the tripeptide GlyAlaCys a. What amino acid is located at the peptides N-terminal end? b. What amino acid is located at the peptides C-terminal end? c. How many peptide bonds are present? d. How many amide linkages are present?arrow_forward22-42 (a) How many atoms of the peptide bond lie in the same plane? (b) Which atoms are they?arrow_forwardConsider the tripeptide leucylvalyltryptophan. a. Specify its structure using three-letter symbols for the amino acids. b. How many peptide bonds are present within the peptide? c. Which of the amino acid residues has the largest R group? d. Which of the amino acid residues, if any, has a basic side chain?arrow_forward
- Consider the tripeptide tyrosylleucylisoleucine. a. Specify its structure using three-letter symbols for the amino acids. b. How many peptide bonds are present within the peptide? c. Which of the amino acid residues has the largest R group? d. Which of the amino acid residues, if any, has an acidic side chain?arrow_forwardWhat special role does the amino acid cysteine have in the peptides vasopressin and oxytocin?arrow_forwardOn complete hydrolysis, a polypeptide gives two alanine, one leucine, one methionine, one phenylalanine, and one valine residue. Partial hydrolysis gives the following fragments: Ala-Phe, Leu-Met, Val-Ala, Phe-Leu. It is known that the first amino acid in the sequence is valine and the last one is methionine. What is the complete sequence of amino acids?arrow_forward
- 22-49 Based on your knowledge of the chemical properties of amino acid side chains, suggest a substitution for leucine in the primary structure of a protein that would probably not change the character of the protein very much.arrow_forward22-89 What kind of noncovalent interaction occurs between the following amino acids? (a) Valine and isoleucine (b) Glutamic acid and lysine (c) Tyrosine and threonine (d) Alanine and alaninearrow_forwardUsing both three- and one-letter codes for amino acids, write the structures of all possible peptides containing the following amino acids: (a) Val, Ser, Leu (b) Ser, Leu2, Proarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





