
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29, Problem 29.48P
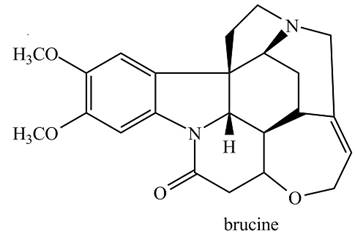
Brucine is a poisonous alkaloid obtained from Strychnos nux vomica, a tree that grows in India, Sri Lanka, and northern Australia. Write out a resolution scheme similar to the one given in Section 29.3A, which shows how a racemic mixture of phenylalanine can be resolved using brucine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Treating chitin with H2O, -OH hydrolyzes its amide linkages, forming a compound called chitosan. What is the structure of chitosan? Chitosan has been used in shampoos, bers for sutures, and wound dressings.
What denuration effect do alkaloid reagents have on proteins?
Give the polarity, melting point, boiling point, solubility, and state at room temperature of the following:
Linoleic acid
Tripalmitin
Triolein
Phosphatidylcholine(28:0)
Phosphatidylinositol(34:1)
Phosphatidylserine(36:2)
Phosphatidylethanolamine(38:6)
Cholesterol
Chapter 29 Solutions
ORGANIC CHEMISTRY
Ch. 29 - Prob. 29.1PCh. 29 - Problem 29.2
What form exists at the isoelectric...Ch. 29 - Problem 29.3
Explain why the of the group of an...Ch. 29 - Prob. 29.4PCh. 29 - Problem 29.5
What -halo carbonyl compound is...Ch. 29 - Problem 29.6
The enolate derived from diethyl...Ch. 29 - Problem 29.7
What amino acid is formed when is...Ch. 29 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 29 - Problem 29.9
Draw the products of each...Ch. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Problem 29.13
What alkene is needed to synthesize...Ch. 29 - Problem 29.14
Draw the structure of each peptide....Ch. 29 - Problem 29.15
Name each peptide using both the...Ch. 29 - Prob. 29.16PCh. 29 - Prob. 29.17PCh. 29 - Problem 29.18
Glutathione, a powerful antioxidant...Ch. 29 - Problem 29.19
Draw the structure of the...Ch. 29 - Problem 29.20
Give the amino acid sequence of an...Ch. 29 - a What products are formed when each peptide is...Ch. 29 - Prob. 29.22PCh. 29 - Devise a synthesis of each peptide from amino acid...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.25PCh. 29 - Consider two molecules of a tetrapeptide composed...Ch. 29 - What types of stabilizing interactions exist...Ch. 29 - Prob. 29.28PCh. 29 - Draw the product formed when the following amino...Ch. 29 - With reference to the following peptide: a...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.32PCh. 29 - Histidine is classified as a basic amino acid...Ch. 29 - Tryptophan is not classified as a basic amino acid...Ch. 29 - What is the structure of each amino acid at its...Ch. 29 - What is the predominant form of each of the...Ch. 29 - 29.37 What is the predominant form of each of the...Ch. 29 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 29 - 29.39 Draw the organic products formed in each...Ch. 29 - 29.40 What alkyl halide is needed to synthesize...Ch. 29 - 29.41 Devise a synthesis of threonine from diethyl...Ch. 29 - 29.42 Devise a synthesis of each amino acid from...Ch. 29 - Prob. 29.43PCh. 29 - Prob. 29.44PCh. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - 29.48 Brucine is a poisonous alkaloid obtained...Ch. 29 - Prob. 29.49PCh. 29 - Prob. 29.50PCh. 29 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 29 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 29 - Prob. 29.53PCh. 29 - Prob. 29.54PCh. 29 - 29.55 Draw the amino acids and peptide fragments...Ch. 29 - Prob. 29.56PCh. 29 - Prob. 29.57PCh. 29 - Prob. 29.58PCh. 29 - 29.59 An octapeptide contains the following amino...Ch. 29 - 29.60 Draw the organic products formed in each...Ch. 29 - Draw all the steps in the synthesis of each...Ch. 29 - 29.62 Write out the steps for the synthesis of...Ch. 29 - 29.63 Besides the Boc and Fmoc protecting groups...Ch. 29 - 29.64 Another method to form a peptide bond...Ch. 29 - 29.65 Draw the mechanism for the reaction that...Ch. 29 - 29.66 Which of the following amino acids are...Ch. 29 - 29.67 After the peptide chain of collagen has been...Ch. 29 - Prob. 29.68PCh. 29 - Prob. 29.69PCh. 29 - 29.70 The anti-obesity drug orlistat works by...Ch. 29 - Prob. 29.71P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 22-9 What is the difference in structure between tyrosine and phenylalanine?arrow_forwardWhat special role does the amino acid cysteine have in the peptides vasopressin and oxytocin?arrow_forward22-42 (a) How many atoms of the peptide bond lie in the same plane? (b) Which atoms are they?arrow_forward
- Why are Guanidins and Amidins such strong bases?arrow_forward(a) Provide four distinct forms of phenylalanine. (b) Rank the solubility of these forms in water. (c) Explain your ranking.arrow_forwardExplain the solubility behavior of each compound in water with relevance to their STRUCTURE. c. Amino Acids and Proteins in Water-Alanine (clear colorless solution)-Glutamic acid (clear colorless solution)-Arginine (clear colorless solution)-Albumin (clear colorless solution)-Casein (clear colorless solution)arrow_forward
- Gramicidin S, a topical antibiotic produced by the bacterium Bacillus brevis, is a cyclic decapeptide formed from five amino acids. Draw the structures of the amino acids that form gramicidin S, and explain why this compound possesses two unusual structural features.arrow_forwardGeneral reactions for drugs belonging to the group of aliphatic amino acids: A) with iodine solution B) With ninhydrin C) With copper sulfate in an alkaline environment D) With resorcinolarrow_forwardShow how to convert the side-chain carboxyl group to a benzyl ester using benzyl chloride as a source of the benzyl group.arrow_forward
- A chemically modified guanidino group is present in cimetidine (Tagamet), a widely prescribed drug for the control of gastric acidity and peptic ulcers. Cimetidine reduces gastric acid secretion by inhibiting the interaction of histamine with gastric H, recep- tors. In the development of this drug, a cyano group was added to the substituted gua- nidino group to alter its basicity. Do you expect this modified guanidino group to be more basic or less basic than the guanidino group of arginine? Explain. N-CN H,C CH,SCH,CH,NHËNHCH, HN, Cimetidine (Tagamet)arrow_forwardCeramide can then be used to synthesize sphingomyelin, another important molecule that is prevalent in cell membranes. 1.How does the structure of sphingomyelin differ from that of ceramide? 2.Sphingomyelin is also found in the cell membrane. Explain how you would expect to find the orientation of this molecule in the cell membrane. 3.Which amino alcohol is used in the synthesis of sphingomyelin? 4.What is particular about the amino alcohol used in in the synthesis of sphingomyelin? 5.How does your answer to #16 affect the physical properties of properties of sphingomyelin?arrow_forwardAnother method to form a peptide bond involves a two-step process Conversion of a Boc-protected amino acid to a p-nitrophenyl ester. Why does a p-nitrophenyl ester “activate” the carboxy group of thefirst amino acid to amide formation?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY