
Determine the number of impaired electrons in each of the following atoms in the ground state and identify each as diamagnetic or paramagnetic: (a) Rb, (b) As, (c) I, (d) Cr, (e) Zn.
(a)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
All the electrons are placed in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
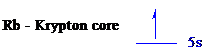
The one electron of
The unpaired electron is present in
(b)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
Arsenic (
The noble gas core for
Put all the 15 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
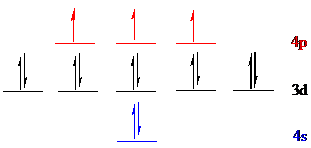
All the 15 electrons of arsenic (
The unpaired electrons are present in
(c)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
Iodine (
The noble gas core for
Put all the 17 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
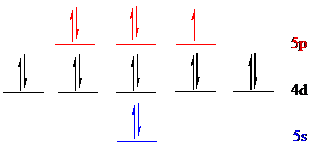
All the 17 electrons of iodine (
The unpaired electron is present in
(d)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule
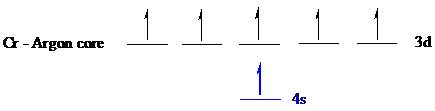
The six electrons of
The unpaired electrons are present in
(e)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.103QP
There is no unpaired electron in
Explanation of Solution
The noble gas core for
Put all the 12 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
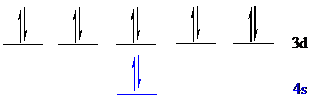
All the 12 electrons of
There is no unpaired electron present in
Want to see more full solutions like this?
Chapter 3 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- 6.9 If a string of decorative lights includes bulbs with wave-lengths of 480, 580, and 700 mm, what are the frequencies of the lights? Use Figure 6.6 to determine which colors are in the set.arrow_forward• identify an orbital (as 1s, 3p, etc.) from its quantum numbers, or vice versa.arrow_forwardThe eyes of certain reptiles pass a single visual signal to the brain when the visual receptors are struck by photons of a wavelength of 850 nm. If a total energy of 3.151014 J is required to trip the signal, what is the minimum number of photons that must strike the receptor?arrow_forward
- Photons are emitted in the Lyman series as hydrogen atoms undergo transitions from various excited states to the ground state. If ground-state He+ are present in the same gas (near stars, for example), can they absorb these photons? Explain.arrow_forwardThe photoelectric work function of potassium is 2.29 eV. A photon of energy greater than this ejects the electron with the excess as kinetic energy. Suppose light of wavelength 455 nm ejects an electron from the surface of potassium. What is the speed of the ejected electron?arrow_forwardThere are an infinite number of allowed electronic transitions in the hydrogen atom. Why dont we see more lines in the hydrogen emission spectrum?arrow_forward
- As the weapons officer aboard the Srarship Chemistry, it is your duty to configure a photon torpedo to remove an electron from the outer hull of an enemy vessel. You know that the work function (the binding energy of the electron) of the hull of the enemy ship is 7.52 1019 J. a. What wavelength does your photon torpedo need to be to eject an electron? b. You find an extra photon torpedo with a wavelength of 259 nm and fire it at the enemy vessel. Does this photon torpedo do any damage to the ship (does it eject an electron)? c. If the hull of the enemy vessel is made of the element with an electron configura tion of [Ar]4s13d10, what metal is this?arrow_forwardUse the mathematical expression for the 2pz wave function of a one-electron atom (see Table 5.2) to show that the probability of finding an electron in that orbital anywhere in the x-y plane is 0. What are the nodal planes for a dxz orbital and for a dx2y2 orbital?arrow_forwardWhat is the threshold frequency for sodium metal if a photon with frequency 6.661014s1 ejects an electron with 7.741020 J kinetic energy? Will the photoelectric effect be observed if sodium is exposed to orange light?arrow_forward
- 6.16 Various optical disk drives rely on laser operating at different wavelengths, with shorter wavelengths allowing a higher density of data storage. For each of the following drive types, find the energy of a single photon at the specified wavelength. (a) CD, =780nm , (b) DVD, =650nm , (c) Blu-ray disc, =405nmarrow_forwardIn 1885, Johann Balmer, a mathematician, derived the following relation for the wavelength of lines in the visible spectrum of hydrogen =364.5 n2( n2 4) where in nanometers and n is an integer that can be 3, 4, 5, . . . Show that this relation follows from the Bohr equation and the equation using the Rydberg constant. Note that in the Balmer series, the electron is returning to the n=2 level.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





