
PRINCIPLES OF INSTRUMENTAL ANALYSIS
7th Edition
ISBN: 9789353506193
Author: Skoog
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 3.19QAP
Interpretation Introduction
Interpretation:
An expression for output voltage should be determined for the circuit shown below.
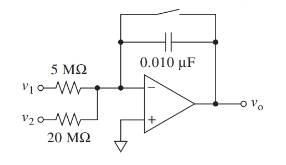
Concept introduction:
The given circuit represents an integrator. Here we have two inputs
Expressing the currents in terms of input and output voltages in the expression gives the final output voltage.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
An electric current of 32.30 A flows for 528. milliseconds. Calculate the amount of electric charge transported.
Be sure your answer has the correct unit symbol and the correct number of significant digits.
Suggest possible reasons for the difference between the experimental and calculated voltages.
n voltammetry the current, which is proportional to concentration is generated by:
Applying a variable current excitation signal
Applying a constant potential excitation signal
Applying a variable potential excitation signal
Chapter 3 Solutions
PRINCIPLES OF INSTRUMENTAL ANALYSIS
Ch. 3 - Prob. 3.1QAPCh. 3 - Prob. 3.2QAPCh. 3 - Prob. 3.3QAPCh. 3 - Prob. 3.4QAPCh. 3 - Prob. 3.5QAPCh. 3 - Prob. 3.6QAPCh. 3 - Prob. 3.7QAPCh. 3 - Prob. 3.8QAPCh. 3 - Prob. 3.9QAPCh. 3 - Prob. 3.10QAP
Ch. 3 - Prob. 3.11QAPCh. 3 - Prob. 3.12QAPCh. 3 - Prob. 3.13QAPCh. 3 - Prob. 3.14QAPCh. 3 - Prob. 3.15QAPCh. 3 - Prob. 3.16QAPCh. 3 - Prob. 3.17QAPCh. 3 - Prob. 3.18QAPCh. 3 - Prob. 3.19QAPCh. 3 - Prob. 3.20QAPCh. 3 - Prob. 3.21QAPCh. 3 - Prob. 3.22QAPCh. 3 - Prob. 3.23QAPCh. 3 - Prob. 3.24QAPCh. 3 - Prob. 3.25QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4)strip serves as the anode and is connected to the positive terminal of a power supply. The negative terminal of the power supply is connected to an insulated copper wire. An ammeter is placed in the circuit and the power supply is turned on. A current of 1.10 A flows for 6 min and 36 sec. During this time the mass loss of the metal is 0.109 g. What is the molar mass of the metal?arrow_forwardThe Weibull distribution is widely used in statistical problems relating to aging of solid insulating materials subjected to aging and stress. Use this distribution as a model for time (in hours) to failure of solid insulating specimens subjected to AC voltage. The values of the parameters depend on the voltage and temperature; suppose ? = 2.2 and ? = 220. (a) What is the probability that a specimen's lifetime is at most 250? Less than 250? More than 300? (Round your answers to five decimal places.) at most 250 less than 250more than 300 (b) What is the probability that a specimen's lifetime is between 100 and 250? (Round your answer to four decimal places.) (c) What value (in hr) is such that exactly 50% of all specimens have lifetimes exceeding that value? (Round your answer to three decimal places.) hrarrow_forwardDirect currents are uniform and have uniform voltage. OTrue O Falsearrow_forward
- A student uses a strip of metal in an electrolysis circuit in which the metal strip serves as the anode and is connected to the positive terminal of a power supply. The negative terminal of the power supply is connected to an insulated copper wire. An ammeter is placed in the circuit and the power supply is turned on. A current of 1.21 A flows for 5 min and 21 sec. During this time the mass loss of the metal is 0.118 g. What is the molar mass of the metal? Answer: 29.317 × (59) g/mol ✔arrow_forwardA student uses a strip of metal in an electrolysis circuit in which the metal strip serves as the anode and is connected to the positive terminal of a power supply. The negative terminal of the power supply is connected to an insulated copper wire. An ammeter is placed in the circuit and the power supply is turned on. A current of 1.21 A flows for 5 min and 21 sec. During this time the mass loss of the metal is 0.118 g. What is the molar mass of the metal?arrow_forwardSuppose a current of 90. A flows through a copper wire for 145. seconds. Calculate how many moles of electrons travel through the wire. Be sure your answer has the correct unit symbol and round your answer to 2 significant digits. ? 2021 McGraw Hill LLC AU Rights Reserved Ts of Uiarrow_forward
- An electric current of 17.70 A flows for 583. milliseconds. Calculate the amount of electric charge transported. Be sure your answer has the correct unit symbol and 3 significant digits. I Don't Know Submit 2022 McGraw Hill LLC All Rights Reserved Lenovoarrow_forwardplease answerarrow_forwardA circuit is constructed with a 6.00 V battery connected across a 2900.0 Ω resistor.(i) Determine the moles of electrons that flow through the circuit per second for 26.98 min.(ii) Determine the number of joules produced by the circuit for the 26.98 min.arrow_forward
- In a wire, 7.23 x 1020 electrons flow past any point during 3.57 s. What is the magnitude I of the current in the wire? Aarrow_forwardThe electric field strength between the plates of a simple air capacitor is equal to the voltage across the plates divided by the distance between them. When a kV voltage of 64.6 V is put across the plates of such a capacitor an electric field strength of 3.4 is measured. Write an equation that will let you calculate the distance d between the plates. Your equation should contain only symbols. Be sure you define each symbol. Your equation: d = 0 Definitions of your symbols: kV 0 = 3.4 cm 0 = 64.6 V 010 X E A cm 3arrow_forwardPlease don't write on a paper. I can't understand handwritten well.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning