
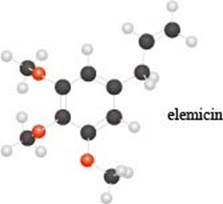
a. Identify the functional groups in the ball-and-stick model of elemicin, a compound partly responsible f or the flavor and fragrance of nutmeg.
b. Draw a skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point.
c. Label all electrophilic carbon atoms.
(a)
Interpretation: The functional groups in the ball-and-stick model of elemicin are to be identified.
Concept introduction: An atom or a group of atoms which are responsible for characteristic physical and chemical properties of the compound are collectively known as functional groups. The functional groups are the most reactive part present in the molecule.
Answer to Problem 31P
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
Explanation of Solution
The given molecule is,
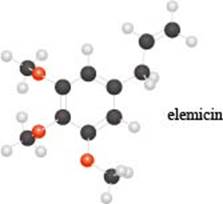
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of elemicin is,
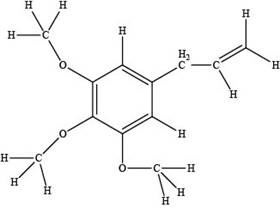
Figure 2
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
(b)
Interpretation: The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is to be drawn.
Concept introduction: The isomers which have same molecular formula but different connectivity of atoms are constitutional isomers.
The temperature at which the vapour pressure of a substance becomes equal to the pressure surrounding the liquid is boiling point. The boiling point depends on the intermolecular forces. Stronger the intermolecular force, higher is the boiling point.
The temperature at which the solid converts to its liquid phase is melting point. Stronger the intermolecular force, higher is the boiling point.
Answer to Problem 31P
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown below.
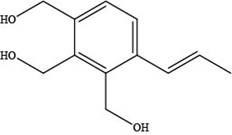
Explanation of Solution
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is,

Figure 3
This structure contains alcohol groups. This structure exhibits intermolecular hydrogen bonding due to the presence of hydrogen atoms bonded to oxygen atom. Hydrogen bonding is strongest intermolecular forces.
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown in Figure 3.
(c)
Interpretation: All electrophilic carbon atoms are to be labeled.
Concept introduction: An electron deficient due to hetero atoms or pi bonds or both is electrophilic site and an electron rich site due to hetero atoms or pi bonds or both is nucleophilic site.
Answer to Problem 31P
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Explanation of Solution
An oxygen atom is more electronegative than carbon atom which makes the carbon atom attached to oxygen atom electron deficient and electrophilic site as shown below.
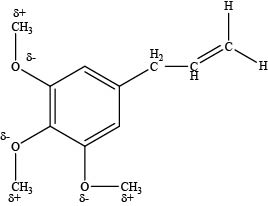
Figure 4
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Want to see more full solutions like this?
Chapter 3 Solutions
Package: Loose Leaf For Organic Chemistry With Connect Access Card (1 Semester)
- Whats the name of step and 1 and drar mechanksm arrows even showing electrons on oxygen atomsarrow_forwarda.Identify the functional groups in the ball-and-stick model of neral, a compound with a lemony odor isolated from lemongrass. b. Draw a skeletal structure of a constitutional isomer of neral that should be more water soluble. c.Label the most electrophillic carbon atom.arrow_forwardConvert each of the attached structures to its more stable chair form. One structure represents menthol and one represents isomenthol. Menthol, the more stable isomer, is used in lip balms and mouthwash. Which structure corresponds to menthol?arrow_forward
- Draw the structures A, B, C, and D.arrow_forwardA. Draw the meantime r of ephedrine. You can use line structures or a hybrid line structure/condense drawing like the one shown. Be sure it is clear which bonds are wedge and which are dash. B. Draw a diastereomer of ephedrine.arrow_forward(a) how many stereoisomers are possible? (b) how many chiral carbons are in the structure? (c) is this optically active?arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




