
Package: Organic Chemistry With Connect 2-semester Access Card
4th Edition
ISBN: 9781259671838
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 3.30P
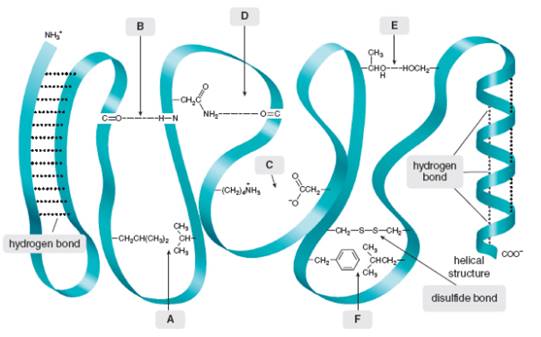
Intramolecular force of attraction are often important in holding large molecule together. For example, some proteins fold into compact, held together by attractive forces between nearby
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Intramolecular forces of attraction are often important in holding large molecules together. For example, some proteins fold into compact shapes, held together by attractive forces between nearby functional groups. A schematic of a folded protein is drawn here, with the protein backbone indicated by a blue-green ribbon, and various appendages drawn dangling from the chain. What types of intramolecular forces occur at each labeled site (A–F)?
Intramolecular forces of attraction are often important in holding largemolecules together. For example, some proteins fold into compactshapes, held together by attractive forces between nearby functionalgroups. A schematic of a folded protein is drawn here, with the proteinbackbone indicated by a blue-green ribbon, and various appendagesdrawn dangling from the chain. What types of intramolecular forcesoccur at each labeled site (A–F)?
The hydrophobic effect is one of the most important noncovalent forces directing the self-assembly of biomolecules in aqueous solution. The hydrophobic effect arises from tendencies of biomolecules (1) to arrange polar groups so that they interact with the aqueous environment by hydrogen bonding and (2) to arrange nonpolar groups so that they are shielded from the aqueous environment. Show how the hydrophobic effect is involved in directing the following.
Q.) The formation of lipid bilayers by phospholipids
Chapter 3 Solutions
Package: Organic Chemistry With Connect 2-semester Access Card
Ch. 3 - Prob. 3.1PCh. 3 - Prob. 3.2PCh. 3 - Draw the structure of a compound fitting each...Ch. 3 - Draw structures that fit each description and name...Ch. 3 - What types of intermolecular forces are present in...Ch. 3 - Prob. 3.6PCh. 3 - Explain why the boiling point of propanamide, is...Ch. 3 - Predict which compound in each pair has the higher...Ch. 3 - Prob. 3.9PCh. 3 - Prob. 3.10P
Ch. 3 - a Label the hydrophobic and hydrophilic portions...Ch. 3 - Prob. 3.12PCh. 3 - Prob. 3.13PCh. 3 - Prob. 3.14PCh. 3 - Prob. 3.15PCh. 3 - Nonactin and valinomycin each contain only two...Ch. 3 - Prob. 3.17PCh. 3 - Problem 3.26 Label the electrophilic and...Ch. 3 - Problem 3.27 Considering only electron density,...Ch. 3 - The fact that sweet-tasting carbohydrates like...Ch. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 3.22PCh. 3 - 3.32 Identify the functional groups in each...Ch. 3 - Draw the seven constitutional isomers having...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 3.27PCh. 3 - Prob. 3.28PCh. 3 - Prob. 3.29PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - Prob. 3.32PCh. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 3.34PCh. 3 - Prob. 3.35PCh. 3 - Explain the observed trend in the melting points...Ch. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Prob. 3.39PCh. 3 - 3.48 Explain why diethylether and have similar...Ch. 3 - Prob. 3.41PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 3.43PCh. 3 - Prob. 3.44PCh. 3 - THC is the active component in marijuana, and...Ch. 3 - Prob. 3.46PCh. 3 - Prob. 3.47PCh. 3 - Prob. 3.48PCh. 3 - Label the electrophilic and nucleophilic sites in...Ch. 3 - By using only electron density arguments,...Ch. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - Prob. 3.53PCh. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - Recall from section 1.10B that there is restricted...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . How many unique amino acid sequences are possible for a tripeptide containing only the amino acids gly, ala, and cys, with each amino acid occurring only once in each molecule?arrow_forwardDraw a segment of the backbone of a protein that is long enough for three peptide linkages to be present.arrow_forward22-59 What is the effect of salt bridges on the tertiary structure of proteins?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY