
(a)
Interpretation: The given compounds in each group are to be ranked in the order of increasing boiling point.
Concept introduction: Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
Intermolecular forces are also known as non-covalent interactions. The interactions present between molecules are known as intermolecular forces. A functional group present in a molecule decides the type of interaction.
Therefore, the increasing order of boiling point is as follows:
Answer to Problem 3.32P
The increasing order of boiling point for the given compounds is
Explanation of Solution
The given compounds are
Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
The increasing order of intermolecular force strength is as follows:
Therefore, the increasing order of boiling point is as follows:
The boiling point increases with an increase in the surface area.
Therefore, the increasing order of boiling point for the given compounds is
The increasing order of boiling point for the given compounds is
(b)
Interpretation: The given compounds in each group are to be ranked in the order of increasing boiling point.
Concept introduction: Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
Intermolecular forces are also known as non-covalent interactions. The interactions present between molecules are known as intermolecular forces. A functional group present in a molecule decides the type of interaction.
Therefore, the increasing order of boiling point is as follows:
Answer to Problem 3.32P
The increasing order of boiling point for the given compounds is
Explanation of Solution
The given compounds are
Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
In
The increasing order of intermolecular force strength is as follows:
Therefore, the increasing order of boiling point is as follows:
Therefore, the increasing order of boiling point for the given compounds is
The increasing order of boiling point for the given compounds is
(c)
Interpretation: The given compounds in each group are to be ranked in the order of increasing boiling point.
Concept introduction: Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
Intermolecular forces are also known as non-covalent interactions. The interactions present between molecules are known as intermolecular forces. A functional group present in a molecule decides the type of interaction.
Therefore, the increasing order of boiling point is as follows:
Answer to Problem 3.32P
The increasing order of boiling point for the given compounds is
Explanation of Solution
The given compounds are
Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
In
The increasing order of intermolecular force strength is as follows:
Therefore, the increasing order of boiling point is as follows:
The boiling point increases with an increase in the surface area. Branched chain alkanes have low boiling point than straight chain alkanes because in branched chain alkanes, surface area is less.
Therefore, the increasing order of boiling point for the given compounds is
The increasing order of boiling point for the given compounds is
(d)
Interpretation: The given compounds in each group are to be ranked in the order of increasing boiling point.
Concept introduction: Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
Intermolecular forces are also known as non-covalent interactions. The interactions present between molecules are known as intermolecular forces. A functional group present in a molecule decides the type of interaction.
Therefore, the increasing order of boiling point is as follows:
Answer to Problem 3.32P
The increasing order of intermolecular force strength for the given compounds is shown below.
![]()
Explanation of Solution
The given compounds are,
![]()
Figure 1
Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
The compounds containing hydroxyl groups show hydrogen bonding.
The increasing order of intermolecular force strength is as follows:
Therefore, the increasing order of boiling point is as follows:
The boiling point increases with an increase in the surface area. Branched chain
Therefore, the increasing order of boiling point for the given compounds is,
![]()
Figure 2
The increasing order of boiling point for the given compounds is shown in Figure 2.
(e)
Interpretation: The given compounds in each group are to be ranked in the order of increasing boiling point.
Concept introduction: Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
Intermolecular forces are also known as non-covalent interactions. The interactions present between molecules are known as intermolecular forces. A functional group present in a molecule decides the type of interaction.
Therefore, the increasing order of boiling point is as follows:
Answer to Problem 3.32P
The increasing order of intermolecular force strength for the given compounds is shown below.
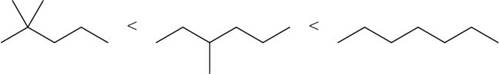
Explanation of Solution
The given compounds are,
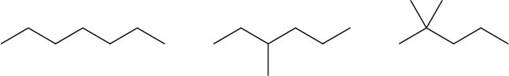
Figure 3
Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
The increasing order of intermolecular force strength is as follows:
Therefore, the increasing order of boiling point is as follows:
The boiling point increases with an increase in the surface area. Branched chain alkanes have low boiling point than straight chain alkanes because in branched chain alkanes, surface area is less.
Therefore, the increasing order of boiling point for the given compounds is,

Figure 4
The increasing order of boiling point for the given compounds is rightfully stated.
(f)
Interpretation: The given compounds in each group are to be ranked in the order of increasing boiling point.
Concept introduction: Boiling point depends upon the intermolecular forces. Greater is the intermolecular forces, greater will be the boiling point.
Intermolecular forces are also known as non-covalent interactions. The interactions present between molecules are known as intermolecular forces. A functional group present in a molecule decides the type of interaction. The increasing order of intermolecular force strength is as follows:
Therefore, the increasing order of boiling point is as follows:
Answer to Problem 3.32P
The increasing order of intermolecular force strength for the given compounds is shown below.
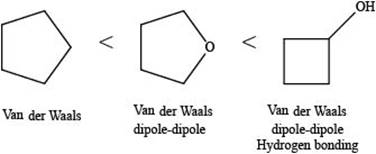
Explanation of Solution
The given compounds are,
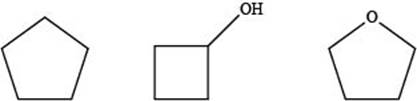
Figure 5
A hydrogen bond is a strong electrostatic attraction which takes place when hydrogen atom is bonded to an electronegative atom (
Dipole-dipole interactions are the forces present between two polar molecules.
Van der Waals forces are the weak forces that are present between non-polar compounds or molecules.
The increasing order of intermolecular force strength is as follows:
The interaction present in cyclopentane is Van der Waals forces because it is a non-polar compound.
The interactions present in cyclobutanolare Van der Waals forces, hydrogen bonding, and dipole-dipole interactions.
Due to electronegativity difference between carbon and oxygen, ethers are polar molecule. Thus, the interaction present in polar molecules is Dipole-dipole interaction. The interactions present in tetrahydrofuran are Van der Waals forces and dipole-dipole interactions.
Therefore, the increasing order of boiling point for the given compounds is,
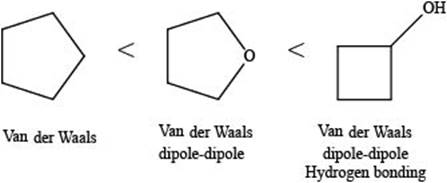
Figure 6
The increasing order of boiling point for the given compounds is rightfully stated.
Want to see more full solutions like this?
Chapter 3 Solutions
Package: Organic Chemistry With Connect 2-semester Access Card
- Rank the compounds NH3, CH4, and PH3 in order of increasing boiling point.arrow_forward5. a) Draw two different structures with the molecular formula C2H6O. b) Name the functional group in each structure c)Which one will have the higher boiling point, and whyarrow_forwardArrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118). (a) CH3CH2OH (b) CH3OCH3 (c) CH3CH2CH3 (d) CH3COOHarrow_forward
- Arrange these compounds in order of increasing boiling point. (a) 1-butanol, butane, diethylether (b) hexane, 1-hexanol, dipropyletherarrow_forwardrank the following compounds in order of decreasing boiling point with 1 having the highest boiling point and 3 having the lowest boiling point. CH3CH2Cl, CH3CCL3, CH3OHarrow_forwardList each set of a compound in order of increasing boiling point.a) hexane, octane, and decanearrow_forward
- Arrange the following in order of increasing boiling points (BP). Explaina) (CH3)3CH b) CH3CH2CH2CH3 c) (CH3)2CHCH3arrow_forwardThe melting points and boiling points of two isomeric alkanes are asfollows: CH3(CH2)6CH3, mp = −57 °C and bp = 126 °C; (CH3)3CC(CH3)3,mp = 102 °C and bp = 106 °C. Explain why there is a small difference in the boiling points of the two compounds, but a huge difference in their melting points.arrow_forwardWhich member in each pair of substances has the higher boiling point? Explain. a) NH3 or PH3 b) CH3CH2OH or CH3CH2CH3 c) HCl or LiCl d) HBr or H2O e) CH3CH2OH or CH3CH2Farrow_forward
- Does CH3OCH3, CH3OH, CH4, CH3CH3, or CH3CH2OH have the highest boiling point?arrow_forwarda. What is the chemical structure of 2,6-dichloroindophenol, circle functional groups differentthan alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? ___________arrow_forwardCyclopentane has a higher boiling point than pentane even though both compounds have the same number of carbons (49 °C vs. 36 °C). Can you suggest a reason for this phenomenon?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





