
Concept explainers
(a)
Interpretation:
The hybridization of each nonhydrogen atom in norethynodrel is to be determined.
Concept introduction:
Answer to Problem 3.38P
| Atom | Hybridization |
| sp |
Explanation of Solution
The structure of Norethynodrel is as below:
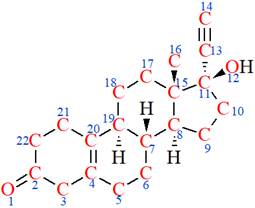
The nonhydrogen atoms are highlighted with red color and numbered.
According to VSEPR theory,
According to VSEPR theory,
According to VSEPR theory, the
According to VSEPR theory,
According to VSEPR theory, geometry of an atom has been determined, and from the geometry of the atom, its hybridization has been determined.
(b)
Interpretation:
The total number of
Concept introduction:
Answer to Problem 3.38P
There are total fifty-one
Explanation of Solution
The structure of Norethynodrel is as below:
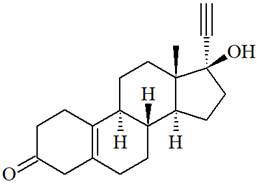
There is one C=C triple bond in norethynodrel molecule. Each triple bond is composed of two
From the
Want to see more full solutions like this?
Chapter 3 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Complete the following table by providing theVSEPR-name and hybridisation (sp, sp2 or sp3) ofthe atom indicated by the arrow.arrow_forwardborazine B3N3H6 is a cyclic compound with alternatig B and N atoms and is isoeletronic with benzene. Identify the hybridization of the N and B atoms. Are there any delocalized electrons in borazine?arrow_forwardGive the (a) bond order and (b) hybridization state of each carbon atom of propadiene, H2CCCH2.arrow_forward
- What are the hybridizations of carbons 1 and 2 respectively in the following structure?arrow_forwardHow many hybrid orbitals are represented in the picture, hoe many s,p,d atomic orbitals corresponding to what hybridization, what electron pair, and what bond angle(s)?arrow_forwardNorethynodrel is a synthetic hormone usedin Enovid, the first oral contraceptive.(a) Determine the hybridization of eachnonhydrogen atom. (b) How many total σbonds and π bonds does norethynodrelhave?arrow_forward
- Determine whether each structure is likely to be colored or not. If colored, indicating the extented conjugation by marking series of continuous sp2 hybridized atomsarrow_forwardAccording to valence bond theory, what is the total number of sp2 hybridized orbitals on all the atoms in formaldehyde, CH2O? Sketch and explain why. According to valence bond theory, how many lone pairs of electrons reside in sp hybridized orbitals in formaldehyde? explain why?arrow_forwardWhich structure(s) has/have a nitrogen atom that is sp3 hybridized?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



