
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
2nd Edition
ISBN: 9780393664034
Author: KARTY
Publisher: NORTON
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 3.37P
Interpretation Introduction
Interpretation:
The total electrons reside in MOs of
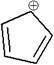
Concept introduction:
Two electrons of a double bond occupy a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
8. How many total electrons reside in MOs of symmetry in the following molecule?
How many symmetry planes and symmetry axis in C3H8O. also is there a center of symmetry in C3H8O?
Draw the complete MO diagram for [CoCl6]4-
Label the AOs and SALCs and MOs.
Draw a sketch for each MO with the appropriate symmetry label
Chapter 3 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
Ch. 3 - Prob. 3.1PCh. 3 - Prob. 3.2PCh. 3 - Prob. 3.3PCh. 3 - Prob. 3.4PCh. 3 - Prob. 3.5PCh. 3 - Prob. 3.6PCh. 3 - Prob. 3.7PCh. 3 - Prob. 3.8PCh. 3 - Prob. 3.9PCh. 3 - Prob. 3.10P
Ch. 3 - Prob. 3.11PCh. 3 - Prob. 3.12PCh. 3 - Prob. 3.13PCh. 3 - Prob. 3.14PCh. 3 - Prob. 3.15PCh. 3 - Prob. 3.16PCh. 3 - Prob. 3.17PCh. 3 - Prob. 3.18PCh. 3 - Prob. 3.19PCh. 3 - Prob. 3.20PCh. 3 - Prob. 3.21PCh. 3 - Prob. 3.22PCh. 3 - Prob. 3.23PCh. 3 - Prob. 3.24PCh. 3 - Prob. 3.25PCh. 3 - Prob. 3.26PCh. 3 - Prob. 3.27PCh. 3 - Prob. 3.28PCh. 3 - Prob. 3.29PCh. 3 - Prob. 3.30PCh. 3 - Prob. 3.31PCh. 3 - Prob. 3.32PCh. 3 - Prob. 3.33PCh. 3 - Prob. 3.34PCh. 3 - Prob. 3.35PCh. 3 - Prob. 3.36PCh. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Prob. 3.39PCh. 3 - Prob. 3.40PCh. 3 - Prob. 3.41PCh. 3 - Prob. 3.42PCh. 3 - Prob. 3.43PCh. 3 - Prob. 3.44PCh. 3 - Prob. 3.45PCh. 3 - Prob. 3.46PCh. 3 - Prob. 3.47PCh. 3 - Prob. 3.48PCh. 3 - Prob. 3.49PCh. 3 - Prob. 3.50PCh. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - Prob. 3.53PCh. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - Prob. 3.56PCh. 3 - Prob. 3.1YTCh. 3 - Prob. 3.2YTCh. 3 - Prob. 3.3YTCh. 3 - Prob. 3.4YTCh. 3 - Prob. 3.5YTCh. 3 - Prob. 3.6YTCh. 3 - Prob. 3.7YTCh. 3 - Prob. 3.8YTCh. 3 - Prob. 3.9YTCh. 3 - Prob. 3.10YTCh. 3 - Prob. 3.11YTCh. 3 - Prob. 3.12YTCh. 3 - Prob. 3.13YT
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a In the Td point group, an S41 improper rotation is equivalent to what other improper rotation? b In the D6h point group, the symmetry operation labeled C21 is equivalent to what other symmetry operation?arrow_forwardDetermine the point groups of the following molecules. a Hydrogen selenide, H2Se b Partially deuterated hydrogen sulfide, or HDS c The chair conformer of cyclohexane, C6H12 d The boat conformer of cyclohexane, C6H12arrow_forwardWhich of the following would be expected to have a dipole moment of zero on the basis of symmetry? Explain. a. SOCl2; b. SiF4; c. OF2.arrow_forward
- Determine the point groups for Chloroethane (staggered conformation).arrow_forwardUsing appropriate diagram(s), show which of the symmetry operation(s) does the [SO4]2- ion have: (i) C4 (ii) σh (iii) S4.arrow_forwardIdentify and Draw the operations, the symmetry elements and how the atoms in the BF3 molecule are transformed by labeling the Fluorine atoms in A, B and C.arrow_forward
- How many total electrons reside in MOs of π symmetry in this cation?arrow_forwardWhat can you conclude about the symmetry of the C3H5+ ion as it actually exists, as regards the bond lengths and the distribution of the positive charge?arrow_forwardDo these molecules has centre of symmetry(C.O.S)? If not,why? If we draw lines passing through the center of the molecule,it meets identical atoms(here both Br atoms and Cl atoms).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Group Theory - Learn like Expert with 3D animation | Introduction for Beginners | ONE Chemistry; Author: One Chemistry;https://www.youtube.com/watch?v=Lz2ih8fkgDs;License: Standard YouTube License, CC-BY