
(a)
Interpretation:
The number of unpaired electrons that is associated with electronic configuration
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
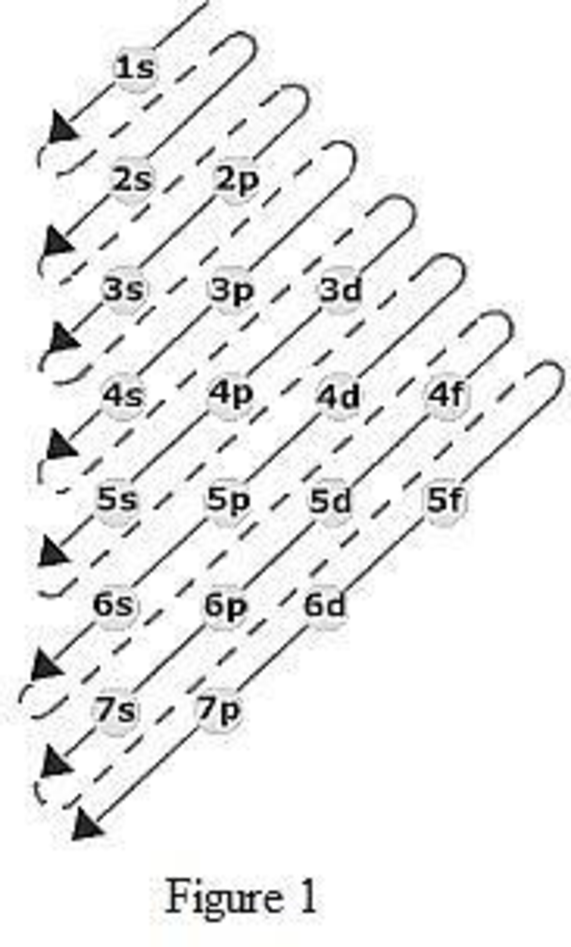
Orbital diagram is the one that gives information about the number of electrons present in the electron orbitals of an atom. The difference between electronic configuration and orbital diagram is that electronic configuration deals with the electrons occupancy in the subshell while orbital diagram deals with the electrons occupancy in the electron orbital. Electrons are paired up in the orbital only when all the orbitals in the subshell are singly filled.
(b)
Interpretation:
The number of unpaired electrons that is associated with electronic configuration
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
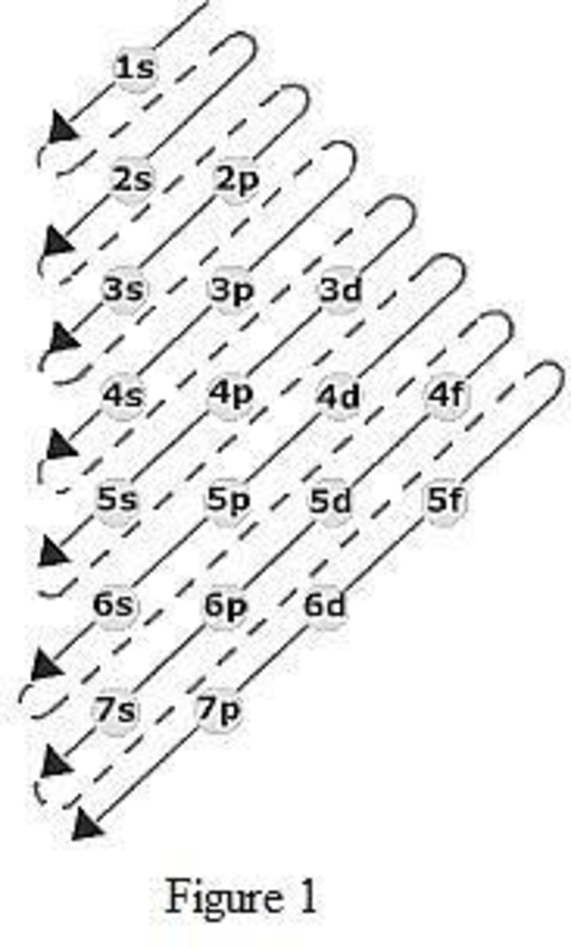
Orbital diagram is the one that gives information about the number of electrons present in the electron orbitals of an atom. The difference between electronic configuration and orbital diagram is that electronic configuration deals with the electrons occupancy in the subshell while orbital diagram deals with the electrons occupancy in the electron orbital. Electrons are paired up in the orbital only when all the orbitals in the subshell are singly filled.
(c)
Interpretation:
The number of unpaired electrons that is associated with electronic configuration
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
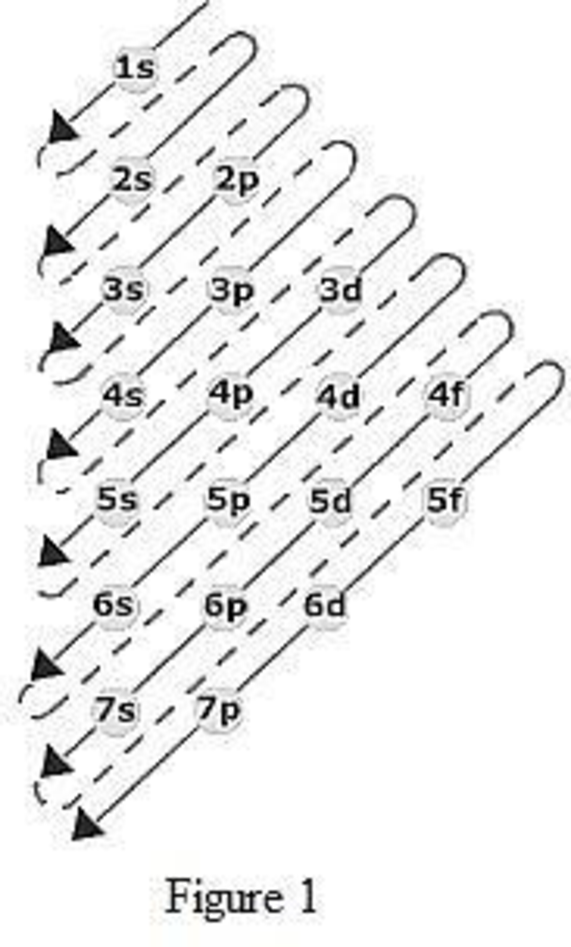
Orbital diagram is the one that gives information about the number of electrons present in the electron orbitals of an atom. The difference between electronic configuration and orbital diagram is that electronic configuration deals with the electrons occupancy in the subshell while orbital diagram deals with the electrons occupancy in the electron orbital. Electrons are paired up in the orbital only when all the orbitals in the subshell are singly filled.
(d)
Interpretation:
The number of unpaired electrons that is associated with electronic configuration
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
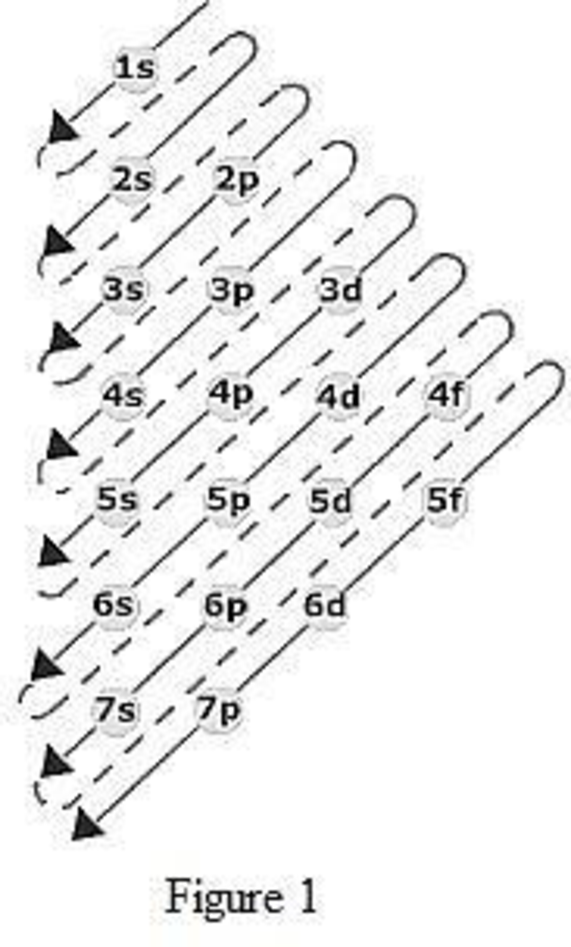
Orbital diagram is the one that gives information about the number of electrons present in the electron orbitals of an atom. The difference between electronic configuration and orbital diagram is that electronic configuration deals with the electrons occupancy in the subshell while orbital diagram deals with the electrons occupancy in the electron orbital. Electrons are paired up in the orbital only when all the orbitals in the subshell are singly filled.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- An excited atom can release some or all of its excess energy by emitting a(n) and thus move to a lower energy state.arrow_forwardCompare the configurations below with two electrons located in p orbitals. Which would be the most stable (have the lowest energy)? Which would be the least stable? Explain your answers.arrow_forwardWhat is the ground-state electron configuration for the oxide ion? A) 1s22p8 B) 1s22s22p2 C) 1s22p6 D) 1s22s22p6 E) 1s22s22p4arrow_forward
- please answer this question correctly. 1. What is the configuration for sulfur after it has achieved stability.arrow_forwardConsider the following electron configurations to answer the questions that follow: (i) 1s2 2s2 2p6 3s1 (ii) 1s2 2s2 2p6 3s2 (iii) 1s2 2s2 2p6 3s2 3p1 (iv) 1s2 2s2 2p6 3s2 3p4 (v) 1s2 2s2 2p6 3s2 3p5 The electron configuration that belongs to the atom with the lowest second ionization energy is ________. (i) (ii) (iv) (iii) (v)arrow_forwardWhich atoms/ions fit the electron configuration? 1s22s22p63s23p64s13d5 Group of answer choices a.None b.V+ c.Mn+ d.Crarrow_forward
- Which of the following elements are generally the most unstable? a. Atomic # 1-20 b. Atomic # 20-43 c. Atomic # 44-83 d. Atomic # 83-118arrow_forwardCompare the configurations below with two electrons located in p orbitals. Which would be the most stable (have the lowest energy)? Which would be the least stable? Explain your answers.</o:p> </v:imagedata> </v:shape></o:p>arrow_forwardWhat is the electron configuration of arsenic (As)?(a) [Ar]4s24p3 (b) [Ar]4s24d104p3 (c) [Ar]4s23d64p3 (d) [Ar]4s23d104p3arrow_forward
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





