
Concept explainers
Recall from section 1.10B that there is restricted rotation around carbon-carbon double
bonds. Maleic acid and fumaric acid are two isomers with vasity different physical properties and
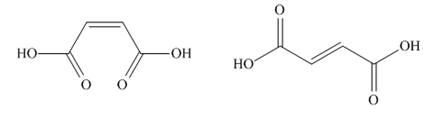
maleic acid fumaric acid
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
ORGANIC CHEMISTRY W/CONNECT & ALEKS
- Aspirin is prepared by the reaction of salicylic- acid with acetic anhydride as shown in the following equation. The stoichiometry of the reaction is given in the equation. Acetic acid is a by-product of the reaction and must be separated and removed so that aspirin can then be sold as a pure product. How many grams of aspirin can be prepared from 120 grams of salicylic acid? Assume that there is an excess of acetic anhydride. (Chapter 4) Acetylsalieylic acid (Aspirin)arrow_forwardWhich of the following gives the proper order for increasing solubility in water? * O Hexane < diethyl ether < butanol O Diethyl ether < hexane < butanol O Butanol < hexane < diethyl ether Diethyl ether < butanol < hexane O Hexane < butanol < diethyl ether octanoic acid is insoluble in water, soluble in NaOH and NaHCO3 O soluble in water and ether O insoluble in water and NaOH, soluble in HCl O insoluble in water, NaOH HCl and H2s04arrow_forwardCream of tartar is a white powder sometimes used in baking. (It's what separates a tangy, chewy snickerdoodle from an ordinary cinnamon-coated sugar cookie. The acid in the cream of tartar gives snickerdoodles their distinctive tangy flavor, and the chew happens because cream of tartar prevents sugar in the cookie dough from crystalizing into crunchiness. Allrecipies.com) Cream of tartar (KHC4H4O6) is the conjugate base salt of tartaric acid (shown to the right). The Ka for another similar acid is 8.7×10-3. What is the standard Gibb's free energy (in kJ/mol) for the dissociation of that other acid?arrow_forward
- 10 Regarding t-butanol and n-butanol, which is/ are correct statements of the following: |- Both are having equal solubility in water Il- t-butanol is more soluble in water than n- butanol III- Boiling point of t-butanol is lower than n-butanol IV- Boiling point of n-butanol is lower than t-butanol * Only I Only II Only II Il & II III & IVarrow_forwardb) Ph NaOH heat, dehydration (CH3)3CCHO KOH heat, dehydration NaOH heat,dehydrationarrow_forwardWhich is NOT a physical property of alcohols or phenols? O Phenols are generally only slightly soluble in water. O The hydroxyl group of an alcohol is nonpolar. The solubilities of normal primary alcohols in water decrease with increasing molecular weight. Boiling points of normal primary alcohols increase with increasing molecular weight.arrow_forward
- Functional Group Classes Alcohol Amide Ester • CH₂CH₂OH * CHIO-CH, 7. H₂C1O Amine Ketone Circle each functional group(s) and identify the class to which each of the groups belongs. 9. H₂C Aldehyde Thiol Aromatics Carboxylic Acid 2. CH,SH 6. Alkenes 4. H₂NCH₂CH₂ 10. Ether NM₂ 11. Which of the functional groups in the top list must be on a terminalC in the carbon chain?arrow_forwardWhy is water more acidic than ethanol but slightly less acidic than methanol?arrow_forward9. An organic compound, P, has the molecular formula, C3H6O. It reacts with chromic acid to form Q. Q reacts with ethanol in the presence of concentrated sulphuric acid to give a sweet-smelling liquid, R. a) Identify P, Q and R. b) Arrange in decreasing order the boiling point of compound P, Q and ethanol. Explain. c) Compare acidity of Q and propanolarrow_forward
- 1. Answer Only True or False? Ketones can be oxidized by Tollen's reagent? True False Refer to the following mixtures: Mixture A: Liquid and solid mixture Mixture B. Liquid mixture whose boiling points are similar. Mixture C. Liquid mixture with high boiling point. Mixture D. Heat sensitive liquid mixture. Which of the following distillation can be applied for Mixture C? vacuum distillation fractional distillation simple distillation none of these In the experiment synthesis of acetyl salicylic acid, what is the purpose of adding water after salicylic acid and acetic anhydride reacted? the destroy the unreacted salicylic acid the destroy the unreacted acetic anhydride to increase the yield of the reaction to dilute the sulfuric acid ————— What smell/odor is expected for the complete reaction of benzyl alcohol and formic acid in the presence of catalytic amount of sulfuric acid? pineapple smell orange smell banana smell wintergreen smell finger nail polish smell…arrow_forwardexplain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forward3. Molecules with more than one alcohol group can react with thionyl chloride (SOCI.) in a way that is different from that of single alcohols. Consider the example below. Provide a reasonable structure for product A CI + 2 HCI OH (product A) онarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


