
Interpretation:
Energy diagram for different conformations of 2,2-dimethylpropane should be sketched and check the resemblance with the energy diagram for ethane and butane.
Concept introduction:
Conformational analysis is the study of energy verses different conformers. Conformers arise due to the free rotation of
Newman projection: Newman projection of molecule is one type of representations for the
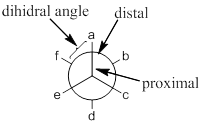
The most and least energy occupied conformers are eclipsed and staggered, which are having a dihedral of ‘0’ and ‘60’ degree respectively.
In ethane all the atoms attached to a carbon atom is same, so the energy levels of different eclipsed conformers and staggered conformers are degenerated.
In butane different atoms are attached to a carbon atom, so the energy levels of eclipsed conformers and staggered conformers are not degenerated.
To find: the energy diagram for conformational analysis of 2,2-dimethylpropane is resemble with the energy diagram for ethane or butane conformations.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





