
Concept explainers
Give the IUPAC name for each compound.
a.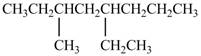 h.
h.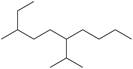 k.
k.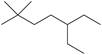
b.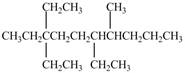 l.
l.![]()
c.
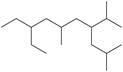 m.
m. 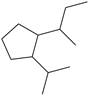
d.
e.
f.
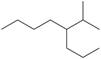 n.
n.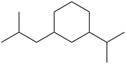
g.
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
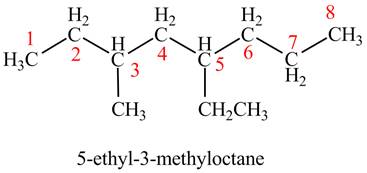
Figure 1
The longest carbon chain has eight carbon atoms. The root word used for eight carbon atoms is oct and the suffix used for
The IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
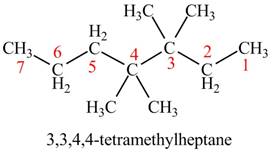
Figure 2
The longest carbon chain has nine carbon atoms. The root word used for nine carbon atoms is dec and the suffix used for
The IUPAC name for the given compound is
(c)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
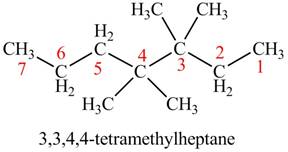
Figure 3
The longest carbon chain has seven carbon atoms. The root word used for seven carbon atoms is hep and the suffix used for
The IUPAC name for the given compound is
(d)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
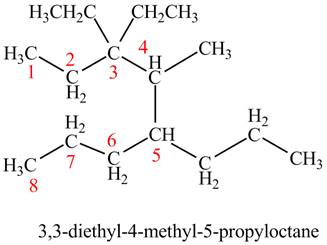
Figure 4
The longest carbon chain has eight carbon atoms. The root word used for eight carbon atoms is oct and the suffix used for
The IUPAC name for the given compound is
(e)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
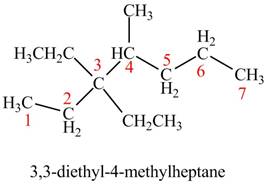
Figure 5
The longest carbon chain has seven carbon atoms. The root word used for seven carbon atoms is hep and the suffix used for
The IUPAC name for the given compound is
(f)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
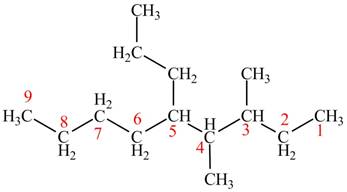
Figure 6
The longest carbon chain has nine carbon atoms. The root word used for eight carbon atoms is non and the suffix used for
The IUPAC name for the given compound is
(g)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
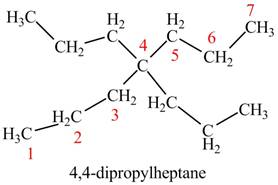
Figure 7
The longest carbon chain has seven carbon atoms. The root word used for seven carbon atoms is hep and the suffix used for
The IUPAC name for the given compound is
(h)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
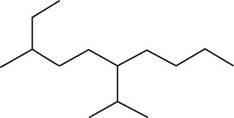
Figure 8
The number of carbon atoms present in longest carbon chain is shown below.
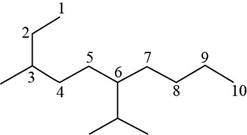
Figure 9
The longest carbon chain has ten carbon atoms. The root word used for ten carbon atoms is dec and the suffix used for
The IUPAC name for the given compound is
(i)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
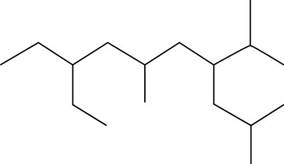
Figure 10
The number of carbon atoms present in longest carbon chain of the given compound is shown below.
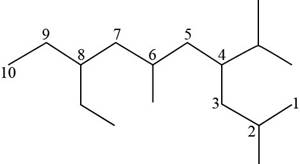
Figure 11
The longest carbon chain has ten carbon atoms. The root word used for ten carbon atoms is dec and the suffix used for
The IUPAC name for the given compound is
(j)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
Figure 12
The longest carbon chain has eight carbon atoms. The root word used for eight carbon atoms is oct and the suffix used for
The IUPAC name for the given compound is
(k)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
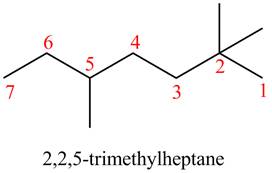
Figure 13
The longest carbon chain has seven carbon atoms. The root word used for seven carbon atoms is hep and the suffix used for
The IUPAC name for the given compound is
(l)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
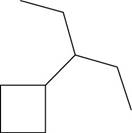
Figure 14
The number of carbon atoms present in longest carbon chain of the given compound is shown below.
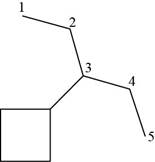
Figure 15
The longest carbon chain has five carbon atoms. The root word used for five carbon atoms is pent and the suffix used for
The IUPAC name for the given compound is
(m)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
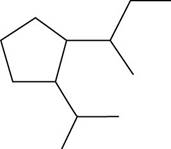
Figure 16
The number of carbon atoms present in longest carbon chain of the given compound is shown below.
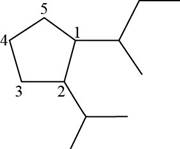
Figure 17
The longest carbon chain has five carbon atoms. The root word used for five carbon atoms is pent and the suffix used for
The IUPAC name for the given compound is
(n)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 4.39P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
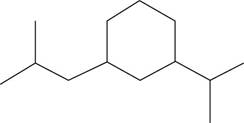
Figure 18
The number of carbon atoms present in longest carbon chain of the given compound is shown below.
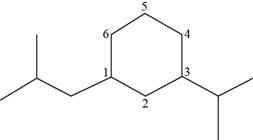
Figure 19
The longest carbon chain has six carbon atoms. The root word used for six carbon atoms is hex and the suffix used for
The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
- Isooctane is the common name of the isomer of C8H18 used as the standard of 100 for the gasoline octane rating: (a) What is the IUPAC name for the compound? (b) Name the other isomers that contain a five-carbon chain with three methyl substituents.arrow_forwardHCl (aq), Zn(Hg) Br2, FeBr3 NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heat CH3Cl, AlCl3 Dilute H3O+ ClCO(CH2)2CH3, AlCl3 NaCCCH2CH3 HNO3, H2SO4 2-butanonearrow_forwardThe skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]arrow_forward
- Draw the following compounds: a) 2-Bromobicyclo[3.3.1]nonane b) 2-Methylbicyclo[2.2.2]octane c) 1,2-Dichlorocyclohexene d) 4,5-Dibromo-1-pentenearrow_forwardi. Why is the boiling point of the aldehyde greater than that of the alkane? ii. Why is the boiling point of alcohol the highest? iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forward1. Give an example of alcohol with structural formula 2. Give an example of phenols with structural formula 3. Give an example of ethers with structural formulaarrow_forward
- Draw an isomer of C5H10O that contains an etherarrow_forwardFour haloalkanes have approximate boiling points of 50°C, 70°C, 90°C and 102°C. Which compound in Figure 4 has an approximate boiling point of 90°C? * A B C Darrow_forwardExplain why CH3CH2NHCH3 has a higher boiling point than (CH3)3N, even though they have the same molecular weight.arrow_forward
- Give iupac name for this diol CH3CH(OH)(CH2)4CH(OH)C(CH3)3arrow_forwardDraw an isomer of C5H10O that contains an alcoholarrow_forwardDraw the structure of a molecule that fi ts each description: a. a 2 ° alcohol of molecular formula C 6H 14O b. an ether with molecular formula C 6H 14O that has a methyl group bonded to oxygen c. a 3 ° alkyl halide with molecular formula C 5H 11Brarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




