
ORGANIC CHEMISTRY-MOLYMOD PACKAGE
5th Edition
ISBN: 9781260227307
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.48P
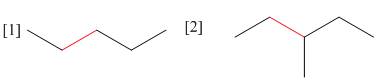
(a) Using Newman projections, draw all staggered and eclipsed conformations that
result from rotation around the bond highlighted in red in each molecule; (b) draw a graph of energy versus dihedral angle for rotation around this bond.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Using Newman projections, draw all staggered and eclipsedconformations that result from rotation around the bond highlighted in red in each molecule; (b) draw a graph of energy versus dihedral angle for rotation around this bond.
HO₂
H
COOH
COOH
V
POH
HOT
COOH
cooff
VI
Ho
HO
COOH
COOH
VI
H
H
COOH
COOH
VIIL
OH
OH
Question: In the structures / examples abovke Identify V, VI, VII and
VIII as Identical enantiomers, ciastereomes, stereo/cumers and/or
structural isomers (there are 6 pairs 7 Comp eand to discover
te I&T I TEN TEM TIMIN).
4. (a) Draw a skeletal (line-bond) structure for 3,4-dimethylhexane.
(b) Draw a sawhorse representation of any staggered conformation of this molecule looking down the
carbon-3 to carbon-4 bond.
(c) Draw a Newman projection looking down the carbon-3 to carbon-4 bond of the same conformation
that you drew as a sawhorse representation.
Chapter 4 Solutions
ORGANIC CHEMISTRY-MOLYMOD PACKAGE
Ch. 4 - Prob. 4.1PCh. 4 - Problem 4.2 Which of the following is not another...Ch. 4 - Problem 4.3 Draw the five constitutional isomers...Ch. 4 - Prob. 4.4PCh. 4 - Prob. 4.5PCh. 4 - Draw the five constitutional isomers that have...Ch. 4 - Problem 4.7 Give the IUPAC name for each...Ch. 4 - Give the IUPAC name for each compound. a....Ch. 4 - Problem 4.9 Give the structure corresponding to...Ch. 4 - Prob. 4.10P
Ch. 4 - Give the IUPAC name for each compound.Ch. 4 - Give the structure corresponding to each IUPAC...Ch. 4 - Arrange the following compounds in order of...Ch. 4 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4 - Prob. 4.15PCh. 4 - Prob. 4.16PCh. 4 - Problem 4.17 a. Draw the three staggered and...Ch. 4 - Problem 4.18 Rank the following conformations in...Ch. 4 - Problem 4.19 Consider rotation around the...Ch. 4 - Calculate the destabilization present in each...Ch. 4 - Problem 4.21 Classify the ring carbons as up or...Ch. 4 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4 - Draw a second chair conformation for each...Ch. 4 - Problem 4.24 Draw both conformations for and...Ch. 4 - Problem 4.25 Draw the structure for each compound...Ch. 4 - For cis-1, 3-diethylcyclobutane, draw a a...Ch. 4 - Prob. 4.27PCh. 4 - Problem 4.28 Consider .
Draw structures f or the...Ch. 4 - Problem 4.29 Draw a chair conformation of...Ch. 4 - Prob. 4.30PCh. 4 - Draw the products of each combustion reaction.Ch. 4 - Explain why beeswax is insoluble in H2O, slightly...Ch. 4 - Prob. 4.33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 - Consider the substituted cyclohexane shown in the...Ch. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - 4.38 Give the IUPAC name for each compound.
a. c....Ch. 4 - 4.39 Give the structure and IUPAC name for each of...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - Prob. 4.41PCh. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 4.43PCh. 4 - Prob. 4.44PCh. 4 - 4.45 Which conformation in each pair is higher in...Ch. 4 - 4.46 Considering rotation around the bond...Ch. 4 - Prob. 4.47PCh. 4 - 4.48 (a) Using Newman projections, draw all...Ch. 4 - 4.49 Label the sites of torsional and steric...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - 4.56 Convert each of the following structures into...Ch. 4 - Prob. 4.57PCh. 4 - Prob. 4.58PCh. 4 - 4.59 Classify each pair of compounds as...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 4.61PCh. 4 - 4.62 Draw the three constitutional isomers having...Ch. 4 - Prob. 4.63PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 4.66PCh. 4 - Prob. 4.67PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 4.69PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 4.71PCh. 4 - Prob. 4.72PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
covered a synthesis of alkynes by a double dehydrohalogenation of dihalides. A student tried to convert trans-2...
Organic Chemistry (9th Edition)
Consider a sample of ideal gas initially in a volume V at temperature T and pressure P. Does the entropy of thi...
General Chemistry: Principles and Modern Applications (11th Edition)
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 3 (a) Draw the two conformations of ethane which has an energy minimum and energy maximum using the Newman projection. Explain why one conformation is lower in energy than the other.arrow_forwardQ2. Answer any TWO of the following parts: (a) Draw the two main conformations that exist for cyclohexane. Explain clearly why one conformer is more stable than the other. Using cis-1-ethyl-3-methylcyclohexane, as an example, explain how ring flipping occurs. Draw both conformers of cis-1-ethyl-3-methylcyclohexane and explain clearly which one predominates. (b) What is polarimetry? The specific rotation of (R)-carvone is - 61°. A chemist prepared a 750 mg mixture of (R)-carvone and its enantiomer in 10 ml of ethanol and placed the solution in a 10 cm polarimeter cell. The observed rotation was - 4.125°. Calculate the specific rotation for the above mixture. What is meant by enantiomeric excess? Then determine the % enantiomeric excess (% ee) in the mixture. (i) (ii) (iii) What % of the mixture is (R)-carvone and (S)-carvone?arrow_forward3)) Draw the most stable chair conformation of a 1,2,4-trimethylcyclohexane (Show all the hydrogens and indicate if the bond is axial or equatorial at the substitution).arrow_forward
- Helparrow_forward1. Draw 5 constitutional isomers of compounds with molecular formula CaHsO2 using expanded or condensed structure. (a)arrow_forwardDraw the Newman projection so that it corresponds to the molecule and conformation shown when viewed down the red bond in the direction of the arrow.arrow_forward
- Draw the most and least stable conformer of chloroethane and 1,2-dibromoethane.arrow_forwardConsider the tricyclic structure B. (a) Label each substituent on the rings as axial or equatorial. (b) Draw B using chair conformations for each sixmembered ring. (c) Label the atoms on the ring fusions (the carbons that join each set of two rings together) as cis or trans to each other.arrow_forwardFor the following two pairs of molecules, (1) Draw out the chair conformation for each molecule, flip the ring if it is possible. (2) Compare both molecules to circle out which one is more stable. (3) Identify their relationship as: constitutional isomer, conformational isomer, stereoisomer or identical. (4) Find all the chiral center on each molecule and label them. VS. ****arrow_forward
- Look at the model of chloroethane. a) Do all chloroethane molecules spend all of their time in this preferred conformation? Explain why or why not. b) What is the preferred conformation called?arrow_forwardSight along the C2-C1 bond of 2-methylpropane (isobutane).(a) Draw a Newman projection of the most stable conformation.(b) Draw a Newman projection of the least stable conformation.(c) Make a graph of energy versus angle of rotation around the C2-C1 bond.(d) Assign relative values to the maxima and minima in your graph, given that an H↔H eclipsing interaction costs 4.0 kJ/mol and an H↔CH3 eclipsing interaction costs 6.0 kJ/mol.arrow_forwardFor the molecule shown below, draw both chair conformations and circle which is the lower energy conformer. .CI "CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License