
ORGANIC CHEMISTRY-MOLYMOD PACKAGE
5th Edition
ISBN: 9781260227307
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.59P
Classify each pair of compounds as constitutional isomers or identical molecules.
a. 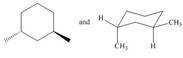 c.
c. 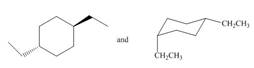
b. 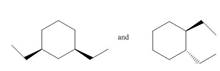 d.
d. 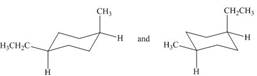
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Give the IUPAC name of the following compound
Determine if each compound is a. constitutional isomers b. enantionmers c. diastereomers d. the same molecule
How are the ball-and-stick models A and B related to each other? Do they represent constitutional isomers or identical molecules?
Chapter 4 Solutions
ORGANIC CHEMISTRY-MOLYMOD PACKAGE
Ch. 4 - Prob. 4.1PCh. 4 - Problem 4.2 Which of the following is not another...Ch. 4 - Problem 4.3 Draw the five constitutional isomers...Ch. 4 - Prob. 4.4PCh. 4 - Prob. 4.5PCh. 4 - Draw the five constitutional isomers that have...Ch. 4 - Problem 4.7 Give the IUPAC name for each...Ch. 4 - Give the IUPAC name for each compound. a....Ch. 4 - Problem 4.9 Give the structure corresponding to...Ch. 4 - Prob. 4.10P
Ch. 4 - Give the IUPAC name for each compound.Ch. 4 - Give the structure corresponding to each IUPAC...Ch. 4 - Arrange the following compounds in order of...Ch. 4 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4 - Prob. 4.15PCh. 4 - Prob. 4.16PCh. 4 - Problem 4.17 a. Draw the three staggered and...Ch. 4 - Problem 4.18 Rank the following conformations in...Ch. 4 - Problem 4.19 Consider rotation around the...Ch. 4 - Calculate the destabilization present in each...Ch. 4 - Problem 4.21 Classify the ring carbons as up or...Ch. 4 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4 - Draw a second chair conformation for each...Ch. 4 - Problem 4.24 Draw both conformations for and...Ch. 4 - Problem 4.25 Draw the structure for each compound...Ch. 4 - For cis-1, 3-diethylcyclobutane, draw a a...Ch. 4 - Prob. 4.27PCh. 4 - Problem 4.28 Consider .
Draw structures f or the...Ch. 4 - Problem 4.29 Draw a chair conformation of...Ch. 4 - Prob. 4.30PCh. 4 - Draw the products of each combustion reaction.Ch. 4 - Explain why beeswax is insoluble in H2O, slightly...Ch. 4 - Prob. 4.33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 - Consider the substituted cyclohexane shown in the...Ch. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - 4.38 Give the IUPAC name for each compound.
a. c....Ch. 4 - 4.39 Give the structure and IUPAC name for each of...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - Prob. 4.41PCh. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 4.43PCh. 4 - Prob. 4.44PCh. 4 - 4.45 Which conformation in each pair is higher in...Ch. 4 - 4.46 Considering rotation around the bond...Ch. 4 - Prob. 4.47PCh. 4 - 4.48 (a) Using Newman projections, draw all...Ch. 4 - 4.49 Label the sites of torsional and steric...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - 4.56 Convert each of the following structures into...Ch. 4 - Prob. 4.57PCh. 4 - Prob. 4.58PCh. 4 - 4.59 Classify each pair of compounds as...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 4.61PCh. 4 - 4.62 Draw the three constitutional isomers having...Ch. 4 - Prob. 4.63PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 4.66PCh. 4 - Prob. 4.67PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 4.69PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 4.71PCh. 4 - Prob. 4.72PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Give one example from main group chemistry that illustrates each of the following descriptions: (a) Covalent ne...
General Chemistry: Atoms First
The smallest building blocks inside your cell phone are about 1000 times smaller than the diameter of a human h...
Chemistry In Context
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Functionalized Hydrocarbons Identify each compound according to its functional group (e.g.,amine,ester,etc.):arrow_forwardWhat types of intermolecular forces are present in each compound?arrow_forwardName each compound and indicate the conformation around the σ bond that joins the two double bonds.arrow_forward
- Classify each carbon-carbon double bond as isolated or conjugatedarrow_forwardIUPAC NAMEarrow_forwardDraw the structure of all compounds that t the following descriptions.a. ve constitutional isomers having the molecular formula C4H8b. nine constitutional isomers having the molecular formula C7H16c. twelve constitutional isomers having the molecular formula C6H12 and containing one ringarrow_forward
- For each of the following compounds, determine whether the cis isomer or the trans isomer is more stable.arrow_forwardGive a clear handwritten answer with explanation..give the IUPAC name of given moleculearrow_forward(b). B. For each of the following, draw a líne structure of a molecule that contains the given functional groups and is consistent with the given molecular formula. Calculate the HDI (hydrogen deficiency index) and label the functional groups.arrow_forward
- Hydrocarbons with only single covalent bond are known as... Select one: O a. alkanes O b. allylenes O C. alkynes O d. alkenesarrow_forwardGlycerol contains: Select one: a. Oxygens double-bonded to carbon, with alkyls on both sides b. oxygens single-bonded to primary and secondary carbons O c. oxygens which are each bonded to two alkyl groups Od. Oxygens double-bonded to carbon, with alkyls on one side only Oe. Oxygens double-bonded to carbon, with an alkyl on one side and an --OH on the other side Clear my choice Previous page F 3 $ 4 % 5 80 MacBook Pro A 6 & 7 8 S Darrow_forward1. Provide the IUPAC name. a. b. H3CO. H₂N D O₂N F NO2 IUPAC name IUPAC namearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY