
Decide whether a precipitate will form when the following solutions are mixed. If a precipitate forms, write a net ionic equation for the reaction.
(a) potassium nitrate and magnesium sulfate
(b) silver nitrate and potassium carbonate
(c) ammonium carbonate and cobalt(lll) chloride
(d) sodium phosphate and barium hydroxide
(e) barium nitrate and potassium hydroxide
(a)
Interpretation:
Whether a precipitate will form when the given solutions are mixed should be determined along with the net ionic equation should be written, if precipitate will form.
Potassium nitrate and magnesium sulfate
Concept introduction:
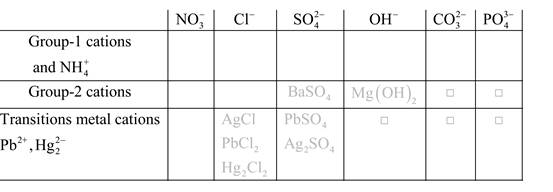
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 7QAP
No precipitation occurs
Explanation of Solution
Potassium nitrate:
Magnesium sulfate:
Reaction for the solution of magnesium nitrate and potassium hydroxide is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
(b)
Interpretation:
Whether a precipitate will form when the given solutions are mixed should be determined along with the net ionic equation should be written, if precipitate will form.
Silver nitrate and potassium carbonate
Concept introduction:
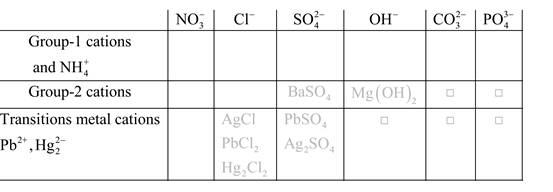
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 7QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Silver nitrate:
Potassium carbonate:
Reaction for the solution of silver nitrate and potassium carbonate is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(c)
Interpretation:
Whether a precipitate will form when the given solutions are mixed should be determined along with the net ionic equation should be written, if precipitate will form.
Ammonium carbonate and cobalt(III) chloride
Concept introduction:
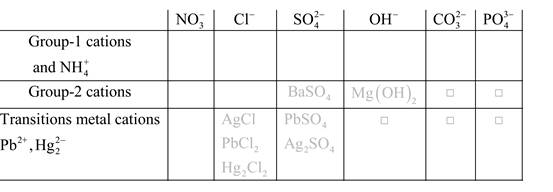
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 7QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Ammonium carbonate:
Cobalt(III) chloride:
Reaction for the solution of ammonium carbonate and cobalt(III) chloride is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(d)
Interpretation:
Whether a precipitate will form when the given solutions are mixed should be determined along with the net ionic equation should be written, if precipitate will form.
Sodium phosphate and barium hydroxide
Concept introduction:
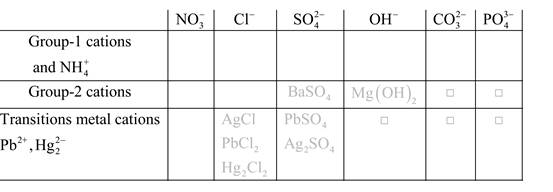
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 7QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Sodium phosphate:
Barium hydroxide:
Reaction for the solution of sodium phosphate and barium hydroxide is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(e)
Interpretation:
Whether a precipitate will form when the given solutions are mixed should be determined along with the net ionic equation should be written, if precipitate will form.
Barium nitrate and potassium hydroxide
Concept introduction:
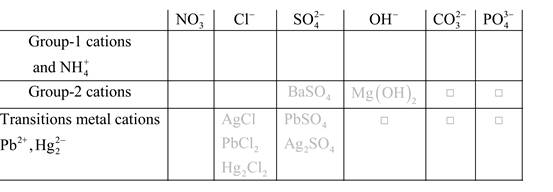
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 7QAP
No precipitation occurs
Explanation of Solution
Barium nitrate:
Potassium hydroxide:
Reaction for the solution of sodium phosphate and potassium hydroxide is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
Want to see more full solutions like this?
Chapter 4 Solutions
Owlv2, 4 Terms (24 Months) Printed Access Card For Masterton/hurley's Chemistry: Principles And Reactions, 8th
- Precipitate Formation ne addition of hydrochloric acid to beakers containing solutions of either sodium chloride (NaCl) or silver nitrate (KNO3) causes a whiteprecipitate in one of the beakers. a. Which beaker contains a precipitate? b. What is the precipitate? c. Write a chemical equation showing the reaction. d. Classify the reaction.arrow_forwardLaws passed in some states define a drunk driver as one who drives with a blood alcohol level of 0.10% by mass or higher. The level of alcohol can be determined by titrating blood plasma with potassium dichromate according to the following equation 16H+(aq)+Cr2O72(aq)+C2H5OH(aq)4Cr3+(aq)+2CO2(g)+11H2O Assuming that the only substance that reacts with dichromate in blood plasma is alcohol, is a person legally drunk if 38.94 mL of 0.0723 M potassium dichromate is required to titrate a 50.0-g sample of blood plasma?arrow_forwardssume a highly magnified view of a solution of HCI that allows you to “see” the HCl. Draw this magnified view. If you dropped in a piece of magnesium, the magnesium would disappear, and hydrogen gas would he released. Represent this change using symbols for the elements, and write the balanced equation.arrow_forward
- Relative solubilities of salts in liquid ammonia can differsignificantly from those in water. Thus, silver bromide issoluble in ammonia, but barium bromide is not (thereverse of the situation in water). Write a balanced equation for the reaction of anammonia solution of barium nitrate with an ammoniasolution of silver bromide. Silver nitrate is soluble inliquid ammonia. What volume of a 0.50 M solution of silver bromidewill react completely with 0.215 L of a 0.076 M solutionof barium nitrate in ammonia? What mass of barium bromide will precipitate fromthe reaction in part (b)?arrow_forwardFollow the directions of Question 7 for solutions of the following: (a) silver nitrate and sodium chloride (b) cobalt(II) nitrate and sodium hydroxide (c) ammonium phosphate and potassium hydroxide (d) copper(II) sulfate and sodium carbonate (e) lithium sulfate and barium hydroxidearrow_forwardWrite the net ionic equation for the reaction, if any, that occurs on mixing (a) solutions of sodium hydroxide and magnesium chloride. (b) solutions of sodium nitrate and magnesium bromide. (c) magnesium metal and a solution of hydrochloric acid to produce magnesium chloride and hydrogen. Magnesium metal reacting with HCl.arrow_forward
- Write a net ionic equation for any precipitation reaction that occurs when 1 M solutions of the following are mixed. (a) copper(II) sulfate and sodium chloride (b) manganese(II) nitrate and ammonium hydroxide (c) silver nitrate and hydrochloric acid (d) nickel(II) sulfate and potassium hydroxide (e) ammonium carbonate and sodium nitratearrow_forwardOn the basis of the general solubility rules given in Table 4.1, predict the identity of the precipitate that forms when the following aqueous solutions are mixed. If no precipitate forms, indicate which rules apply.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





