
Concept explainers
(a)
An expression for the probability that an electron in ground state will be found outside a sphere of radius
(a)
Answer to Problem 78AP
The expression for the probability that an electron in ground state will be found outside a sphere of radius
Explanation of Solution
Write the expression for the radial probability density function for the Hydrogen atom in ground state.
Here,
Write the expression to find the probability to for an electron in grounds state to be found outside a sphere of radius
The probability outside a sphere is to be found. So integration must be done from radius of sphere to infinity.
Use expression (I) in (III) to find
Integrate expression (IV) by integration by parts.
Simplify expression (V) to find
Conclusion:
Apply limits from
Therefore, the expression for the probability that an electron in ground state will be found outside a sphere of radius
(b)
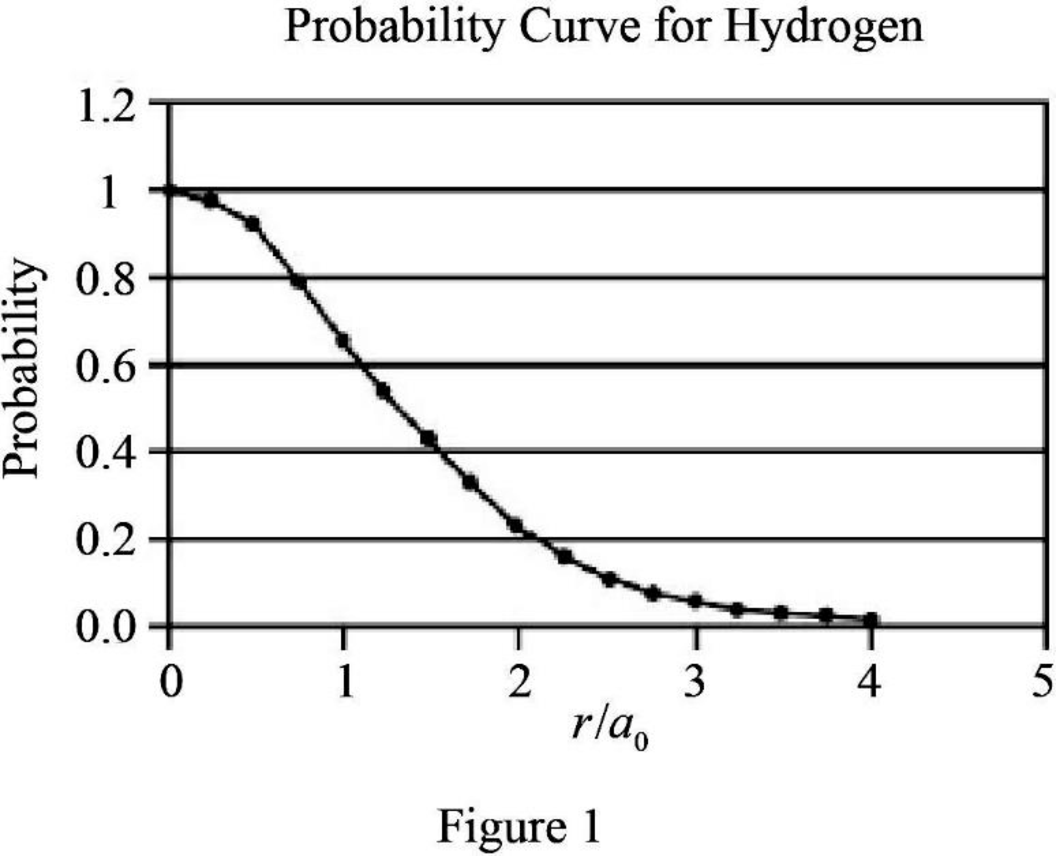
Graph the relation between probability and
(b)
Answer to Problem 78AP
The graph between probability and
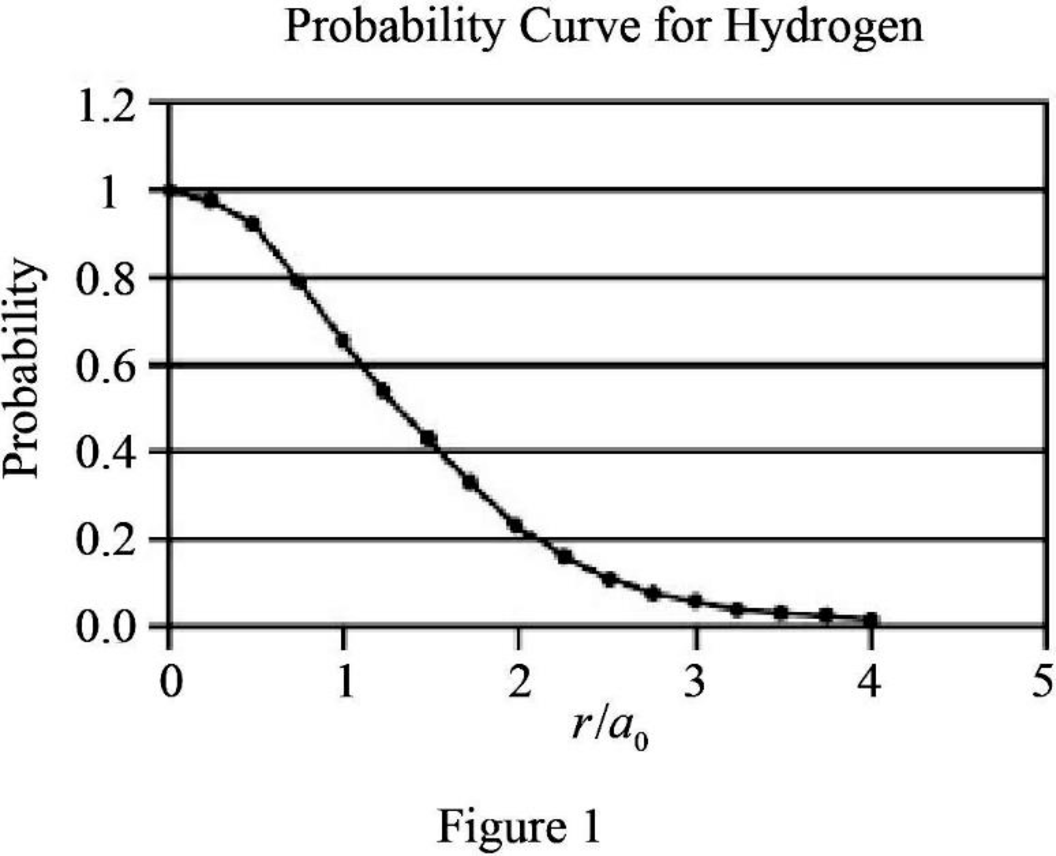
Explanation of Solution
The expression for probability as a function of
The expression is exponential in nature. The graph will be exponentially decreasing as the value of
Assume values between
Figure 1 below shows the plot between probability and
Conclusion:
Therefore, The graph between probability and
(c)
The value of
(c)
Answer to Problem 78AP
The value of
Explanation of Solution
The probability to detect an electron inside or outside a sphere of radius
Use expression for probability derived in part (a).
Substitute
Put
Conclusion:
Equation (VIII) is a transcendental equation. Solving it the value of
Therefore, the value of
Want to see more full solutions like this?
Chapter 42 Solutions
Physics: for Science.. With Modern. -Update (Looseleaf)
- Explain how a hydrogen atom in the ground state (l = 0) can interact magnetically with an external magnetic field.arrow_forward(a) Show that if you assume the average nucleus is spherical with a radius r=r0A1/3, and with a mass at A u, then its density is independent at A. (b) Calculate that density in u/fm3 and kg/m3, and compare your results with those found in Example 31.1 for 56Fe.arrow_forwardWhat are the possible polar orientations of the spin momentum vector for an electron?arrow_forward
- Justify the following hypothesis by photoelectric effect: “For each metal, there exists a minimum binding energy for an electron characteristic of the element, called the work function (W0).arrow_forward(a) For a given value of the principal quantum number n for a hydrogen atom, how many values of the orbital quantum number l are possible? (b) For a given value of , how many values of the orbital magnetic quantum number ml are possible? (c) For a given value of n, how many values of ml are possible?arrow_forwardConsider the electron of a Li2+ ion that undergoes a transition from a higher energy state n to its adjacent lower energy state n – 1 (e.g. n = 2→1, 3→2, 4→3, etc) and emits a photon. Suppose the emitted photon is used to strike the surface of potassium, which has a threshold frequency of 5.464 × 10^14 s–1.a) Whatisthemaximuminitialquantumnumber,n, that is required in order to emit a photon with high enough energy to generate a photocurrent from the metal surface?b) Usingthenvaluesolvedinpart(a), calculate the maximum speed of the photoelectron from potassium. If you couldn’t solve for n in part (a), use n = 3.arrow_forward
- Using the Bohr formula for the radius of an electron orbit,estimate the average distance from the nucleus for anelectron in the innermost n=1 orbit of a uranium atom(z=92)Approximately how much energy would berequired to remove this innermost electron?arrow_forward(a) Assuming it is nonrelativistic, calculate the velocity of an electron with a 0.100-fm wavelength (small enough to detect details of a nucleus). (b) What is unreasonable about this result? (c) Which assumptions are unreasonable orinconsistent?arrow_forward
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax





