
Concept explainers
(a)
Interpretation: The missing formal charges in below set of resonance structure should be added. Also, unimportant resonance structure should be determined.
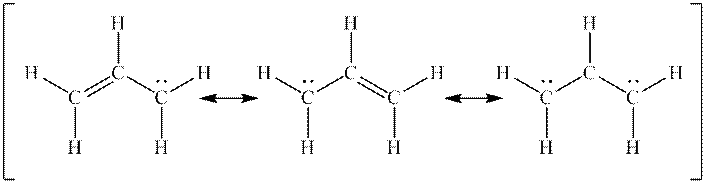
Concept introduction: Lewis structure is representation of molecule in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of electrons in the molecule.
When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(b)
Interpretation: The missing formal charges in below set of resonance structure should be added. Also, unimportant resonance structure should be determined.
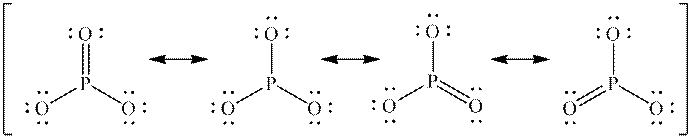
Concept introduction: Lewis structure is representation of molecule in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of electrons in the molecule.
When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge.
2. Use only arrow type 3 to move a positive charge for resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Trending nowThis is a popular solution!

Chapter 5 Solutions
Custom eBook for Organic Chemistry
- For the following Structure, a) draw in any missing lone pairs; b) identify and list the resonance patterns (i.e. allylic cation) present. If one pattern shows up more than once, list it multiple times c) draw curved arrows on the initial structure to produce a resonance structure, including formal charge. (hint - use a minimum of curved arrows for each resonance structure). Repeat on the second structure to generate a third structure. Rank the resonance structures in order of decreasing importance: 1 is most important, 2 is second most important, etc... H Pattern 1: Pattern 2: Rank: Pattern 1: Pattern 2: Rank: Pattern 1: Pattern 2: Rank:arrow_forwardAdd curved arrows to show how the first resonance structure can be converted to the second.arrow_forwardYou are given bond-line structures (which means that not all of the hydrogens are shown) and all nonzero formal charges are given. Locate and fill in all lone pair electrons in the structures.arrow_forward
- For the reaction given below, draw a mechanism (curved arrows) and then predict which side of the reaction is favoured under equilibrium conditions. Include lone pairs and formal charges.arrow_forwardneed it fast Find the formal charge on each atom in the following resonance structure.arrow_forwardIn the following Lewis structure of [(CH3)2OH]*, every atom, bond and lone pair is positioned. To complete the structure, drag the formal charge tags to the appropriate atom(s). Each marker may be used more than once, or not at all. If an atom has a formal charge of zero, do not drag a tag to it. When you drag the marker in, place the little crosshairs in the upper left corner of the marker directly over the atom(s) in question (not above them). H. H-C Н-С-О-С-Н C-H H HH 2- II 2-arrow_forward
- Add curved arrows to show how the first resonance structure can beconverted to the second.arrow_forwardFor each structure below (1) show all resonance forms (do not increase overall # of formal charges), (2) label the resonance form that is the highest contributor, (3) draw the resonance hybrid. It may be helpful to insert hydrogens and lone pair electrons! NH2arrow_forward11. Add curved arrows to the following structures to show how electron pairs must be moved to interconvert the structures, and locate any formal charges. This is the nitrate ion and it has three resona onance structures (RS). וך- :ó: :0: 1. supply formal charges 1. supply formal charges 2. draw e pushing arrows draw third RS here 2. draw e¯ pushing arrows that make second RS to make third RSarrow_forward
- This question requires you to show arrow pushing! Draw all lone pairs on the 5) 6 molecule below first and next draw at least six resonance structures upon delocalization of appropriate electrons. show formal charges on each resonance structure NH2arrow_forwardDraw all resonance structures for the following compounds. Include curved www arrows NH₂arrow_forwardDraw the Lewis structure of the missing product. Make sure to include lone pairs and non-zero formal charges.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
