
(a)
Interpretation:
The number of 4d electrons in Cd should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
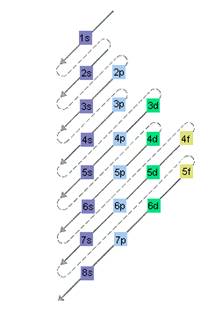
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
(b)
Interpretation:
The number of 4p electrons in Br should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
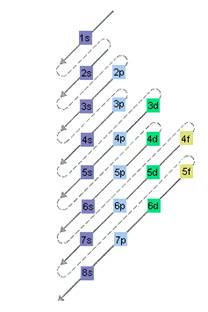
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
(c)
Interpretation:
The number of 6p electrons in Bi should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
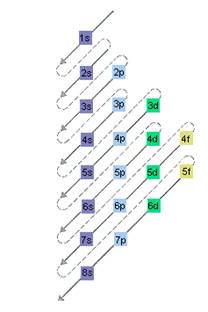
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
(d)
Interpretation:
The number of 4s electrons in Zn should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
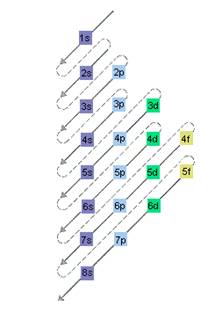
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Basic Chemistry (5th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





