
ORGANIC CHEMISTRY TEXT PACKAGED - 2 YE
10th Edition
ISBN: 9781260024241
Author: Carey
Publisher: MCG/CREATE
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 30P
The reaction of cyclopentyl bromide with sodium cyanide to give cyclopentyl cyanide
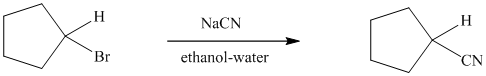
proceeds faster if a small amount of sodium iodide is added to the reaction mixture. Can you suggest a reasonable mechanism to explain the catalytic function of sodium iodide?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
] The reaction of 2-bromopropane and sodium ethoxide in ethanol reacts 6.7 times faster than 2-bromo-1-deuteriopropane under the same conditions. Explain what mechanism this data is consistent with, and why
1.) Establish the reaction mechanism for the conversion of 2,3-dimethylbutanol to the corresponding alkyl halide if hydrochloric acid is used. What method of chemical reaction would you use? Justify your answer. Show the overall reaction and the reaction mechanism.
If the rate of reaction of [0.1 M] sodium cyanide with [0.1 M] 2-bromo-2-methylpropane is 1.2 mole/second, what would be the effect on theoverall rate if the concentration of sodium cyanide is increased to [0.2 M] and the concentration of the alkyl bromide is decreased to [0.05 M]?
Chapter 6 Solutions
ORGANIC CHEMISTRY TEXT PACKAGED - 2 YE
Ch. 6.1 - Prob. 1PCh. 6.2 - 1-Bromo-3-chloropropane reacts with one molar...Ch. 6.3 - Prob. 3PCh. 6.3 - The Fischer projection for (+)-2-bromooctane is...Ch. 6.3 - Would you expect the 2-octanol formed by SN2...Ch. 6.3 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - The first step in the synthesis of the...Ch. 6.6 - Prob. 9PCh. 6.6 - Prob. 10P
Ch. 6.7 - Prob. 11PCh. 6.8 - Prob. 12PCh. 6.9 - Diethyl ether (CH3CH2OCH2CH3) has a dielectric...Ch. 6.9 - Unlike protic solvent which solvate from complexes...Ch. 6.10 - Prob. 15PCh. 6.10 - Prob. 16PCh. 6.10 - The hydrolysis of sulfonate of 2-octanol is...Ch. 6.11 - Prob. 18PCh. 6 - Prob. 19PCh. 6 - Prob. 20PCh. 6 - Both of the following reactions involve...Ch. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Sodium nitrite (NaNO2) reacted with 2-iodooctane...Ch. 6 - Prob. 25PCh. 6 - Prob. 26PCh. 6 - Prob. 27PCh. 6 - The reaction of 2,2-dimethyl-1-propanol with HBr...Ch. 6 - If the temperature is not kept below 25oC during...Ch. 6 - The reaction of cyclopentyl bromide with sodium...Ch. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - Write an equation, clearly showing the...Ch. 6 - Prob. 34PCh. 6 - Based on what we know about nucleophiles and...Ch. 6 - Prob. 36PCh. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41DSPCh. 6 - Prob. 42DSPCh. 6 - Prob. 43DSPCh. 6 - Prob. 44DSPCh. 6 - Prob. 45DSPCh. 6 - Prob. 46DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two mechanisms are among those that have been postulated for decomposition of aryl diazonium salts in aqueous solution containing nucleophilic anions, A—:arrow_forwardImagine that phenol (“hydroxybenzene”) and nitrobenzene are reacted (in separate beakers) with a hot solution containing both concentrated sulfuric acid and concentrated nitric acid. A) Phenol reacts much more quickly than benzene, and benzene reacts much more quickly that nitrobenzene. Explain this observation, using at least one appropriate reaction coordinate diagram as part of your answer (be sure to label your reaction diagram with appropriate structures). You do not need to include any complete mechanisms, but you may wish to use portions of mechanisms as part of your discussion.arrow_forwardImagine that phenol (“hydroxybenzene”) and nitrobenzene are reacted (in separate beakers) with a hot solution containing both concentrated sulfuric acid and concentrated nitric acid. A) In analyzing the products, you discover that the substitution pattern resulting from the reaction with phenol differs from the reaction with nitrobenzene. Explain this difference in substitution patterns using one or more judiciously selected reaction mechanisms or portions of mechanisms.arrow_forward
- Consider the mechanism for the formation of a halohydrin from cyclohexene and bromine in the presence of water to form (1S,2S)-2-bromocyclo-1-hexanol. Part A: Describe in full detail what you think is happening on the molecular level for this reaction. Be sure to discuss the role of each reactant and intermediate. Part B: Using a molecular level explanation, explain why this reaction occurs. Be sure to discuss why the reactants form the products shown.arrow_forwardUsing clear illustrations, discuss the main differences between E1 and E2 mechanisms in organic reactions.arrow_forwardOptically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below: 1. What are the stereochemical designations (R or S) of Compound 1 and Compound 2? 2. On the basis of the structure of Compound 2 and the information on the reaction conditions, suggest which type of mechanism Compound 1 undergoes . 3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction. 4. draw a mechanism on a piece of paper (using curly arrows) to show the formation of Compound 2 from Compound 1 including any activated complex. 5. If the sodium methoxide is left out of the reaction mixture, Compound 2 is formed in roughly equal amounts with another compound (Compound 3). Suggest a structure for Compound 3. With reference to the mechanism of the reaction and the structure of Compound 1, explain how these…arrow_forward
- Give the mechanism of the following reactions. Briefly explain why any selectivities occur.arrow_forwardWrite the mechanism for the Friedel Crafts Acylation reaction of ethylbenzene using acetyl chloride and aluminum chloride. What ratio of ortho-, para-, and meta- products would you expect? Explain why you expect that ratio.arrow_forwardWrite the mechanism of the reaction given below. Mention clearly the name and class of reaction give in 20 minutearrow_forward
- Suggest reasonable mechanisms for the following reactions:arrow_forwardWrite the mechanism for the following reactions :arrow_forwardWrite the mechanism and predict the product for the reaction of cyclohexene with bromine. Make sure that you include the stereochemistry for the reaction in your mechanism. Then describe in detail the appearance of the reactants and products in this reaction. What would be the visible evidence that a reaction took place? Explain paragraph why no color change would occur upon mixing cyclohexanol with a solution of bromine.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License