
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 42P
Interpretation Introduction
Interpretation:
Factors affecting the rate of a reaction needs to be determined.
Concept introduction:
Enthalpy,
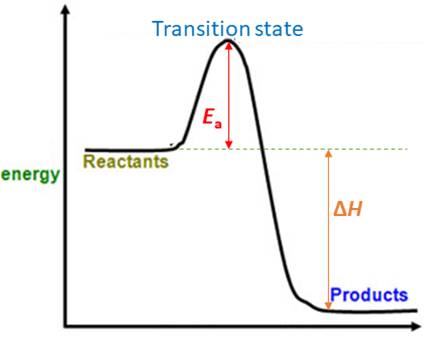
Energy difference between the reactants and the transition state is known as activation energy ( Ea , see the figure below). Activation energy, Ea is the minimum energy required by the reactants to initiate a reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The sugar glucose (C6H12O6) is broken down in a decomposition reaction to ethanol (C2H6O) and bubbles, CO2. Write the balanced chemical equation for the reaction.
5. What is meant by the phrase “reaction to completion”?
C3H60 + H30+ are the reactants what are the products?
Chapter 6 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 6.1 - Prob. 6.1PPCh. 6.1 - Prob. 6.1PCh. 6.1 - Prob. 6.2PPCh. 6.1 - Prob. 6.2PCh. 6.2 - Using the values in Table 6.2, give H for each...Ch. 6.2 - Prob. 6.4PPCh. 6.2 - Answer the following questions using the given...Ch. 6.2 - Given the H and balanced equation in Sample...Ch. 6.2 - Prob. 6.6PPCh. 6.3 - Prob. 6.7PP
Ch. 6.4 - Consider the reaction of ozone (O3) with nitrogen...Ch. 6.4 - Draw an energy diagram for an uncatalyzed...Ch. 6.5 - Identify the forward and reverse reactions in each...Ch. 6.5 - Write the expression for the equilibrium constant...Ch. 6.5 - Consider the reversible reaction AB, with K=1....Ch. 6.5 - Given each equilibrium constant, state whether the...Ch. 6.5 - Consider the following reaction:...Ch. 6.5 - Using the equilibrium mixture of reactants and...Ch. 6.5 - Calculate the equilibrium constant for each...Ch. 6.5 - Consider the representation depicted in the...Ch. 6.6 - Prob. 6.13PPCh. 6.6 - Prob. 6.14PPCh. 6.6 - wThe conversion of H2O to H2 and O2 is an...Ch. 6.6 - The reaction of O2 with NO to form NO2 and O2 is...Ch. 6.6 - wIn which direction is the equilibrium shifted in...Ch. 6.6 - Label each statement about the following...Ch. 6 - Prob. 11PCh. 6 - Prob. 12PCh. 6 - Prob. 13PCh. 6 - Prob. 14PCh. 6 - Prob. 15PCh. 6 - Prob. 16PCh. 6 - Prob. 17PCh. 6 - Prob. 18PCh. 6 - Prob. 19PCh. 6 - Prob. 20PCh. 6 - Prob. 21PCh. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Prob. 24PCh. 6 - Prob. 25PCh. 6 - Prob. 26PCh. 6 - Prob. 27PCh. 6 - Ammonia ( NH3 ) decomposes to hydrogen and...Ch. 6 - Prob. 29PCh. 6 - Ethanol ( C2H6O ), a gasoline additive, is formed...Ch. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - Prob. 33PCh. 6 - Prob. 34PCh. 6 - Draw an energy diagram for the following reaction...Ch. 6 - Prob. 36PCh. 6 - State two reasons why increasing temperature...Ch. 6 - Why does decreasing concentration decrease the...Ch. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Which of the following affect the rate of a...Ch. 6 - Prob. 42PCh. 6 - How does a catalyst affect each of the following:...Ch. 6 - What is the difference between a catalyst and an...Ch. 6 - Prob. 45PCh. 6 - Consider the representation depicted in the...Ch. 6 - For each value, are the reactants or products...Ch. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Prob. 51PCh. 6 - Consider three different equilibrium mixtures...Ch. 6 - Write an expression for the equilibrium constant...Ch. 6 - Write an expression for the equilibrium constant...Ch. 6 - Prob. 55PCh. 6 - Use each expression for the equilibrium constant...Ch. 6 - Prob. 57PCh. 6 - Consider the following reaction:...Ch. 6 - Prob. 59PCh. 6 - Which of the following representations ([1][3]) of...Ch. 6 - Consider the following reaction....Ch. 6 - Consider the following reaction. H2(g)+I2(g)2HI(g)...Ch. 6 - Prob. 63PCh. 6 - Prob. 64PCh. 6 - Consider the reaction of N2(g)+O2(g)2NO(g). What...Ch. 6 - Consider the reaction of H2(g)+F2(g)2HF(g). What...Ch. 6 - Prob. 67PCh. 6 - Consider the reversible reaction ABA+B, shown at...Ch. 6 - Consider the endothermic conversion of oxygen to...Ch. 6 - Consider the exothermic reaction:...Ch. 6 - Consider the exothermic reaction:...Ch. 6 - Consider the endothermic reaction:...Ch. 6 - Consider the gas-phase reaction of ethylene...Ch. 6 - Methanol (CHO), which is used as a fuel in race...Ch. 6 - Prob. 75PCh. 6 - How does a catalytic converter clean up automobile...Ch. 6 - Prob. 77PCh. 6 - The reaction of salicylic acid with acetic acid...Ch. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83CPCh. 6 - Prob. 84CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reusable booster rockets of the space shuttle use a mixture of aluminum and ammonium perchlorate as fuel. A possible reaction is 3Al(s)+3NH4ClO4(s)Al2O3(s)+AlCl3(s)+3NO(g)+6H2O(g) Calculate H for this reactionarrow_forwardHow is the addition of heat symbolized in a chemical equation? The addition of light energy?arrow_forwardThe carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward
- list at least three quantities that must be conserved in chemical reactions.arrow_forward4.80 The reaction shown below is used to destroy Freon-12 (CF2Cl2), preventing its release into the atmosphere. What mass of NaF will be formed if 250.0 kg of CF2Cl2 and 400.0 kg of Na2C2O4 are heated and allowed to react to completion? CF2Cl2+2Na2C2O42NaF+2NaCl+C+4CO2arrow_forward4.64 Using the web, find information about the amount of lead in the environment during the past 50 years. Correlate what you observe with the presence or absence of tetraethyl lead in gasoline.arrow_forward
- Many cereals are made with high moisture content so that the cereal can be formed into various shapes before it is dried. A cereal product containing 58% H2O by mass is produced at the rate of 1000. kg/h. What mass of water must be evaporated per hour if the final product contains only 20.% water?arrow_forward4.60 Why are fuel additives used?arrow_forwardThe solubility of BaSO4 in water is 2.33×10−3 gm/litre. Its solubility product will be (molecular weight of BaSO4=233) equalarrow_forward
- If the amount of sugar dissolved in water is found to be 65.1797 g/L, what is this concentration in kg/kLarrow_forwardWrite the products or reactants.arrow_forwardHydrogen cyanide is produced industrially from reactions; ammonia gas, oxygen and methane, with the following equation: 2NH3 (g) + 3O2 (g) + 2CH4 (g) → 2HCN (g) + 6H2O (g) Calculate how many kg of cyanide acid can be produced from the reaction of ammonia, oxygen and methane gas with a mass of 5.00 x 103 kg each, if the reaction efficiency is 100%?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY