
Concept explainers
(a)
Interpretation: The migrating carbon atom and curved arrow for migration are interpreted for the given carbocation rearrangement.
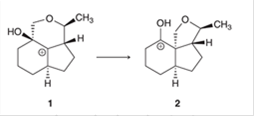
Concept introduction: The chemical transformation can be shown by the arrow pushing method in which the curved arrows show the movement of electrons or protons. A carbocation is one of the intermediates that can rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary > Secondary > Primary
(b)
Interpretation: The more stability of carbocation (2) compared to (1) is interpreted for the given carbocation rearrangement.
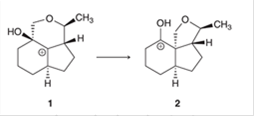
Concept introduction: The chemical transformation can be shown by the arrow pushing method in which the curved arrows show the movement of electrons or protons. A carbocation is one of the intermediates that can rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary > Secondary > Primary
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY LL PRINT UPGRADE
- 6. Rank the alkenes in order of increasing stability. (1 = least stable, 4 = most stable)arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. OH CH3 + + H3C CH₂ CH3 First stage in synthesis of the preservative BHT • You do not have consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one. ▼ H3PO4 ? OH CH3 CH3 CH3 CH3arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. OH CCI3 H2SO4 CCI3 CI- First stage in synthesis of the insecticide DDT, which is now banned in the US You do not have to consider stereochemistry. Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.arrow_forward
- Draw the least stable resonance form for the intermediate in the following electrophilic substitution reaction. NO₂ C Br₂ / FeBr3 Br • You do not have to consider stereochemistry. Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one. NO₂arrow_forwardQuestion 6 (1 point) Which is the most stable carbocation? C A OA B.arrow_forward9:32 52% Organic Chemistry for Biology Students ... Chemistry for Biology Students) 24 What is the final product of the 5 ?following reaction sequence (ähä 1) D CH,=CH. H* 2) KMuO,, A OH CH=CH, CHCH, CO.H CH,CH, CH,CO,H d. a b. d.arrow_forward
- 10:19 the reaction of 1-butyne with the four hydrogen atoms result .A CH3-CH2-CH2-CH3 .B CH2=CH-CH2-CH3 .C CH3-CH2-CH=CH2 .D CH3-CH=CH-CH3arrow_forward8.18a Modify the given carbon skeleton to draw the major product of the given reaction. Use the single bond tool to interconvert between double and single bonds. 1) Вн, THF 2) HO, NaOH H,C CH2 * Edit Drawing Save for Later Attempts: 0 of 15 used Submit Answer 3:04 PM P Type here to search 15°F A G 40 ENG 2/5/2022arrow_forward6. a. b. C. PRINT the name of the following alkenes. Specify E/Z were necessary.arrow_forward
- 4. Carbocations can undergo rearrangements to form more stable carbocations. A fourmembered ring can expand to a five-membered ring and a five-membered ring can expand to a six-membered ring, but a six-membered ring will not expand to a seven-membered ring. Provide an explanation for why the expansion of four- and five-membered rings is favorable, but the expansion of six-membered rings is not. O ** Carrow_forward7. Rank the following alkenes from 1-4. 1 being most stable and 4 being least stable.arrow_forward12. (10 pts) Under the right conditions radical bromination of A produces compound B as shown below (this is termed allylic bromination): hv Br Br2 A B Write a detailed mechanism for the propagation steps of the above radical bromination, showing how the products are formed and that the radical used in the first step is formed in the last step (use curved arrows to show electron flow).arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
