
Concept explainers
(a)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, σ bond or the region where the bond is formed if the new bond is a π bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
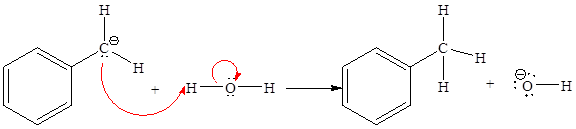
Explanation of Solution
The given proton transfer reaction is
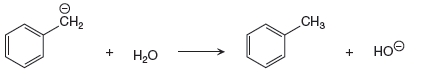
In the above reaction, the bond
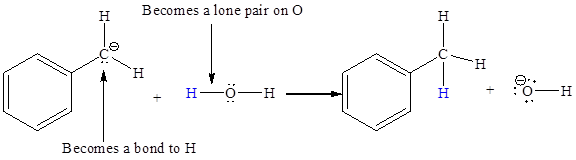
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on C to the H on water (highlighted blue) to illustrate the formation of
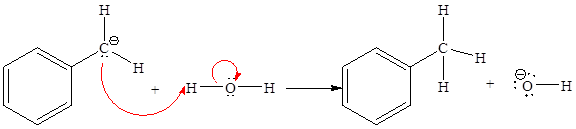
The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
(b)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond.
bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
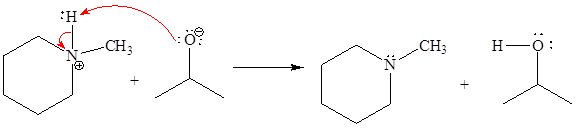
Explanation of Solution
The given proton transfer reaction is
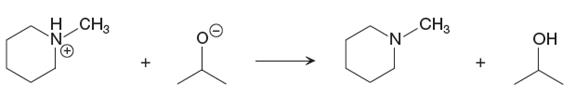
In the above reaction, the bond
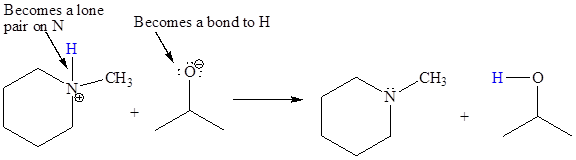
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on O to the H on N (highlighted blue) to illustrate the formation of the

The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
(c)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond.
bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
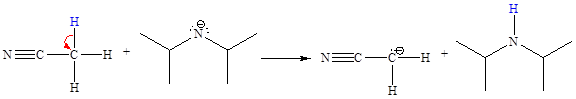
Explanation of Solution
The given proton transfer reaction is
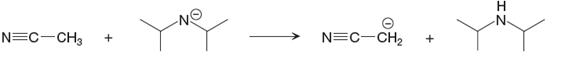
In the above reaction, the bond
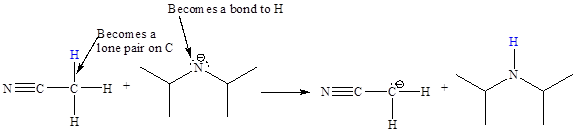
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on N to the H on C (highlighted blue) to illustrate the formation of the
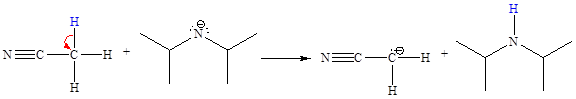
The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
(d)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond.
bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
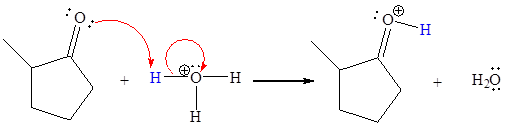
Explanation of Solution
The given proton transfer reaction is
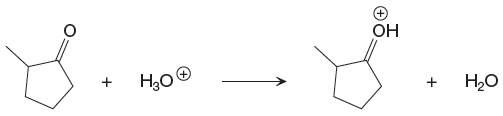
In the above reaction, the bond
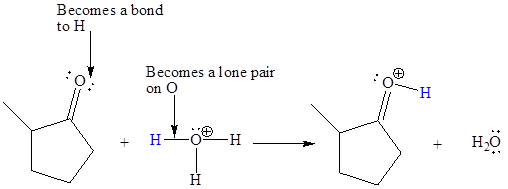
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on O to the H of
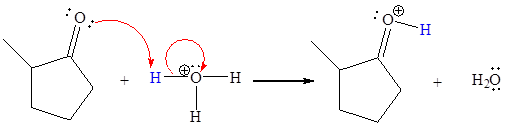
The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
Want to see more full solutions like this?
Chapter 6 Solutions
ORGANIC CHEMISTRY:PRIN...(PB)-W/ACCESS
- can you answer this question with an explaination please?arrow_forwardDraw the product of the Lewis acid-base reaction shown in the picture attached and include formal charges/lone pairs. Draw any curly arrows to show electron flowarrow_forwardFor each reaction, label the Lewis acid and base. Use curved arrow notation to show the movement of electron pairs.arrow_forward
- Can you please help how to answer this question with elaborate step?arrow_forwardFor each attached reaction, label the Lewis acid and base. Use curved arrow notation to show the movement of electron pairs.arrow_forwardSolve correctly please, with some explanation also. Draw the product(s) for the following radical reaction where one bond breaks and another bond is formed. The curved arrows indicate the movement of an unpaired electron.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

